by Stephanie Baum , Medical Xpress
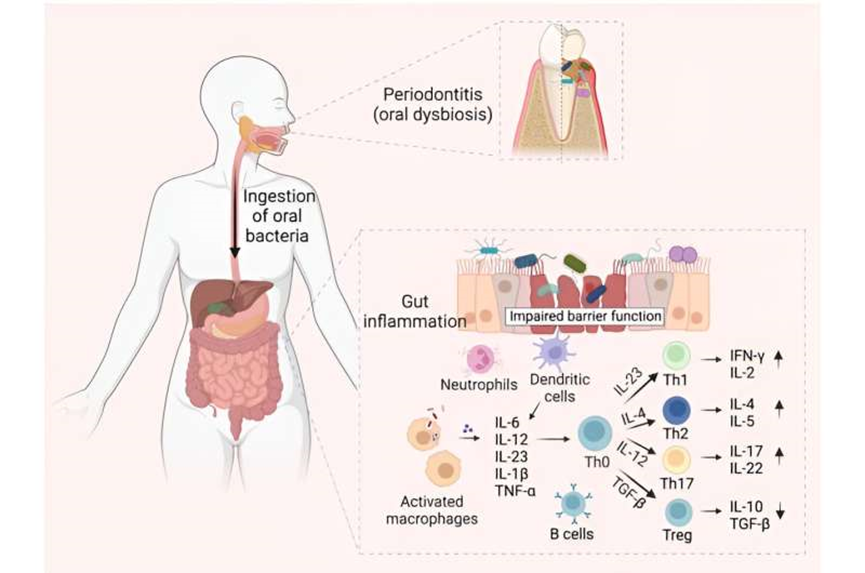 Oral pathogen-mediated immune responses that drive gut inflammation in IBD. Periodontitis onset triggers expansion of pathogenic oral bacteria. Constant saliva swallowing enables these bacteria to translocate to the gut. A compromised intestinal barrier function, marked by diminished mucus and epithelial barrier integrity, facilitates the penetration of oral bacteria into the sub-epithelial regions. Neutrophils, the first responders, attempt to phagocytose the ingested bacteria and release antimicrobial substances. Additionally, the antigen-presenting cells (APCs) in the gut recognize the microbial invaders through pattern recognition receptors like Toll-like receptors (TLRs). Once activated, these cells release a cocktail of pro-inflammatory molecules: interleukin (IL)-6, IL-12, IL-23, IL-1β, tumor necrosis factor α (TNF-α), and chemokines. Consequently, these molecules guide the differentiation of naive T helper (Th0) cells into Th1, Th2, Th17, and Treg cells. Furthermore, B cells become activated by recognizing bacterial antigens and differentiate into plasma cells, which produce antibodies specific to the oral bacterial antigens. These antibodies neutralize or opsonize bacteria and can form immune complexes, intensifying inflammation. The concerted actions of innate and adaptive immune cells lead to the initiation or exacerbation of inflammatory process in IBD, highlighting the far-reaching effects of oral bacterial dysbiosis. Figure created with BioRender.com. Credit: arXiv (2023). DOI: 10.48550/arXiv.2308.10907
Oral pathogen-mediated immune responses that drive gut inflammation in IBD. Periodontitis onset triggers expansion of pathogenic oral bacteria. Constant saliva swallowing enables these bacteria to translocate to the gut. A compromised intestinal barrier function, marked by diminished mucus and epithelial barrier integrity, facilitates the penetration of oral bacteria into the sub-epithelial regions. Neutrophils, the first responders, attempt to phagocytose the ingested bacteria and release antimicrobial substances. Additionally, the antigen-presenting cells (APCs) in the gut recognize the microbial invaders through pattern recognition receptors like Toll-like receptors (TLRs). Once activated, these cells release a cocktail of pro-inflammatory molecules: interleukin (IL)-6, IL-12, IL-23, IL-1β, tumor necrosis factor α (TNF-α), and chemokines. Consequently, these molecules guide the differentiation of naive T helper (Th0) cells into Th1, Th2, Th17, and Treg cells. Furthermore, B cells become activated by recognizing bacterial antigens and differentiate into plasma cells, which produce antibodies specific to the oral bacterial antigens. These antibodies neutralize or opsonize bacteria and can form immune complexes, intensifying inflammation. The concerted actions of innate and adaptive immune cells lead to the initiation or exacerbation of inflammatory process in IBD, highlighting the far-reaching effects of oral bacterial dysbiosis. Figure created with BioRender.com. Credit: arXiv (2023). DOI: 10.48550/arXiv.2308.10907
Though oral health issues can affect overall health, the two are considered unrelated and are frequently addressed separately when it comes to treatment. However, existing research shows that patients with inflammatory bowel disease (IBD) are more likely to have periodontitis and vice-versa, suggesting an "oral-gut axis" linking the two conditions reciprocally. As such, collaborative, holistic health care may serve as an effective approach for such patients.
A new comprehensive review of over 300 studies examining the correlation of periodontitis and IBD adds support to the argument. The work, titled "Unraveling the Link between Periodontitis and Inflammatory Bowel Disease: Challenges and Outlook," is published on the arXiv pre-print server.
Periodontitis is the advanced stage of gum disease, characterized by inflamed and receding gums, halitosis, deep periodontal pockets, loose teeth, and if left untreated, tooth loss. Bacterial plaque accumulations on teeth or in the spaces between them result in an inflammatory response in the supporting structure of teeth, eventually destroying it.
As the most common cause of tooth loss in adults worldwide, periodontitis is found in approximately half of adults over age 30 and over 70% of adults over age 65. Risk factors for periodontitis include advanced age, diabetes, genetic factors, certain medications, poor oral hygiene, and smoking. The spread of the disease's pathogens and the systemic inflammation it promotes is believed to contribute to other health issues including diabetes, heart disease, IBD, and preterm birth.
Meanwhile, Crohn's disease and ulcerative colitis are the two main forms of IBD, a broad category covering a number of chronic inflammatory issues that affect the GI tract. In Crohn's disease, patches of inflammation may be found on the bowel wall throughout both the large and small intestines, while inflammation in ulcerative colitis is found on the mucosal layer from the colon to the rectum.
In both of these IBD conditions, symptoms include abdominal pain and cramping, diarrhea, fatigue, fever, nausea and vomiting, and weight loss. Oral lesions are among the significant comorbidities seen in anywhere from 25% to 40% of patients with Crohn's disease and ulcerative colitis.
The exact cause of IBD has not been identified, but environmental, genetic, and microbial factors as well as immune responses are all believed to be involved. Risk factors may include age (it is more common in children and young adults, but also affects up to 15% of patients after age 60); ethnicity (it more commonly appears in Caucasians and Ashkenazi Jews); family history, and specifically for Crohn's disease, smoking.
A number of oral symptoms, including gingivitis (the first stage of gum disease) and periodontitis, have been shown to affect up to 30% of IBD patients either before, after, or at the same time as GI issues begin. Though the reason for this has not been fully elucidated, the factors of genetics, immune system dysregulation, and changes in the oral microbiome are all believed to play a part.
However, though evidence points to a relationship between IBD and periodontitis, its causality and directionality are still unclear. Existing research shows that a specific sequence of events may be necessary to precipitate development of IBD associated with periodontitis. The new review outlines these events and examines the inflammatory responses that the two conditions share, proposing a "multi-hit model" by which periodontitis and its related oral bacteria may be implicated.
Looking forward, the review notes the need for longitudinal studies to illustrate in more detail the connection between the two conditions. Notably, in conclusion it asserts that there must be collaboration among health care professionals and specialists including dentists, gastroenterologists, immunologists and infectious disease experts/microbiologists to provide holistic oral-systemic health care, as well as the need for reduction of pathogenic oral bacteria to the gut via outstanding dental care.
Researchers from the University of Maryland School of Dentistry, the Icahn School of Medicine at Mount Sinai, The Forsyth Institute, the University of Turin, the University of Minnesota, the University of Maryland School of Medicine, and Johns Hopkins University School of Medicine contributed to this review.







Post comments