For the first time spatial omics leads to a lifesaving treatment
What’s spatial medicine? We’re not talking about space medicine or going to Mars, or a form of rehab. This is about a landmark paper from the Max Planck Institute of Biochemistry, leading an international collaboration across many countries (authors and work from Germany, Japan, Denmark, China, Australia, France, Switzerland, and USA), that moved the field from spatial biology as a research tool to medicine as a therapy. I’ve coined this term to reflect the importance of this advance.

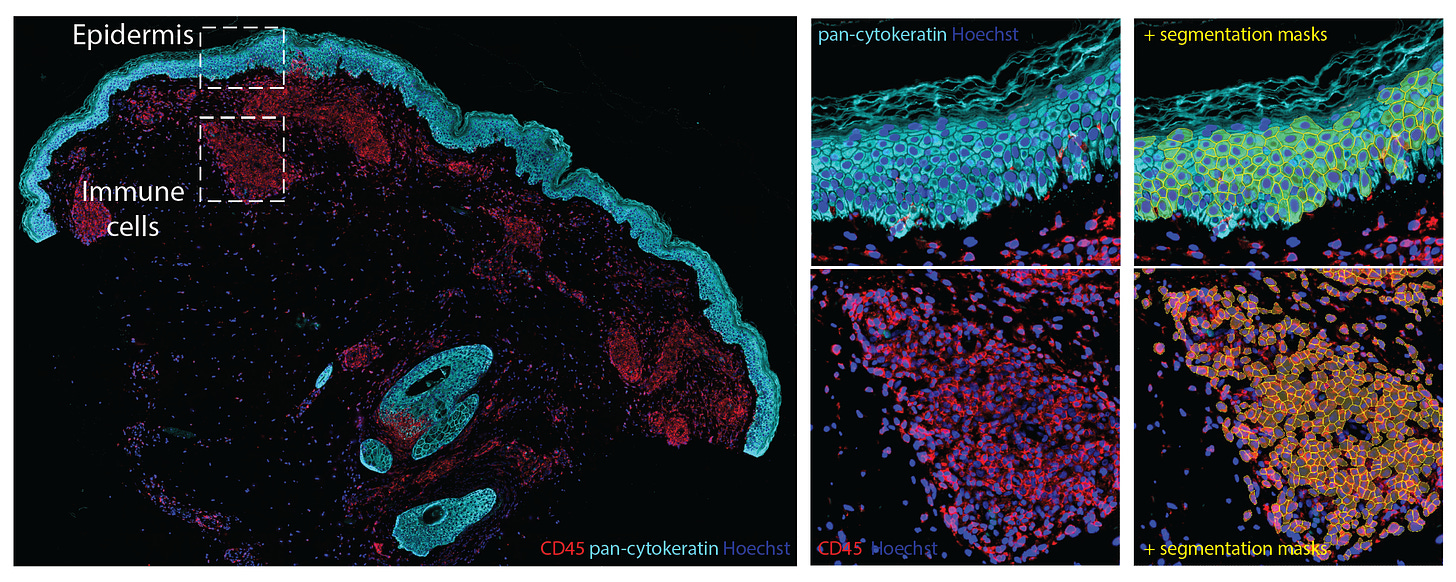
An overview spatial map image made by Georg Walmann at Max Planck Institute of Biochemistry. The “shoreline” is the epidermis and distinct cellular types within the dermis inner layer are tagged, involved with the life-threatening condition of toxic epidermal necrolysis.
Brief Background on Spatial Biology
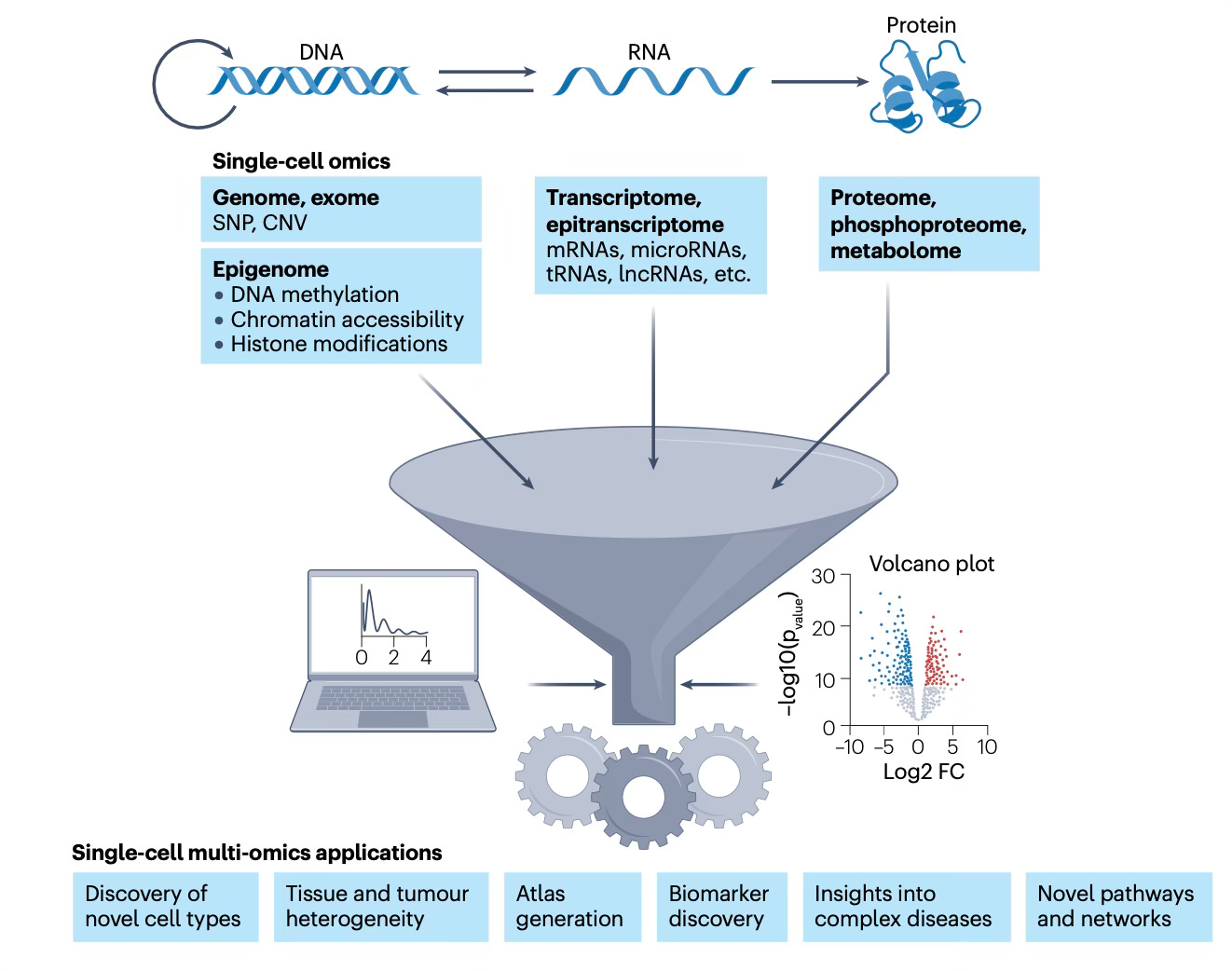
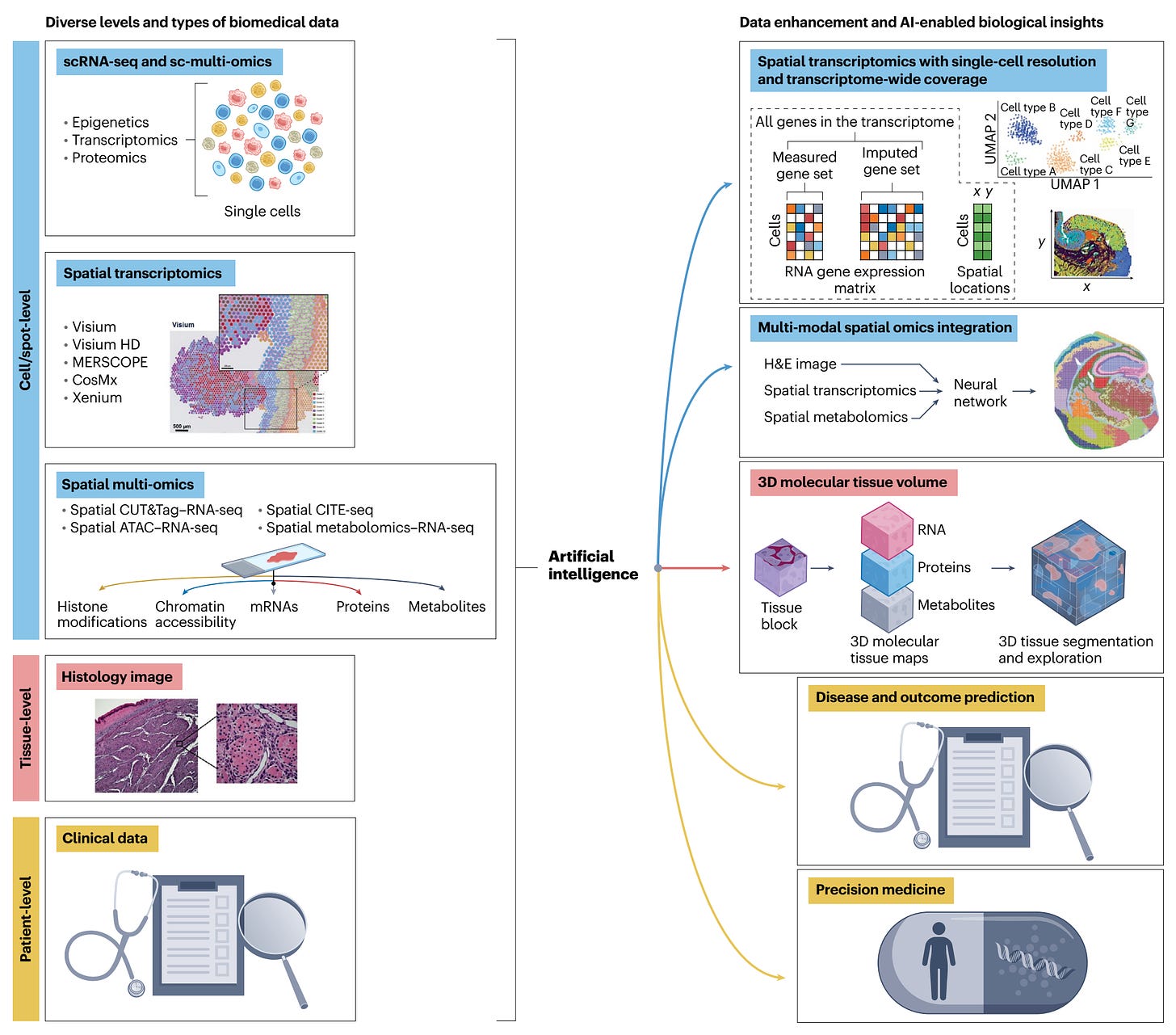
I’ve previously written about spatial biology here, one of, if not the hottest area in life science, providing exquisite spatiotemporal maps of cells, nuclei, or cellular constituents. Spatial transcriptomics (RNA-sequencing) was heralded in 2020 by Nature Methods as “The Method of the Year,” but has since rapidly expanded to spatial multi-omics (Figure summary below) with massive throughputs that get at the cellular level genome, proteome, metabolite, and epigenome. Combining the omic biologic data with imaging provides spatial resolution. That relies on machine learning and A.I. analytic tools. Some of the many applications are listed at the bottom of the Figure.
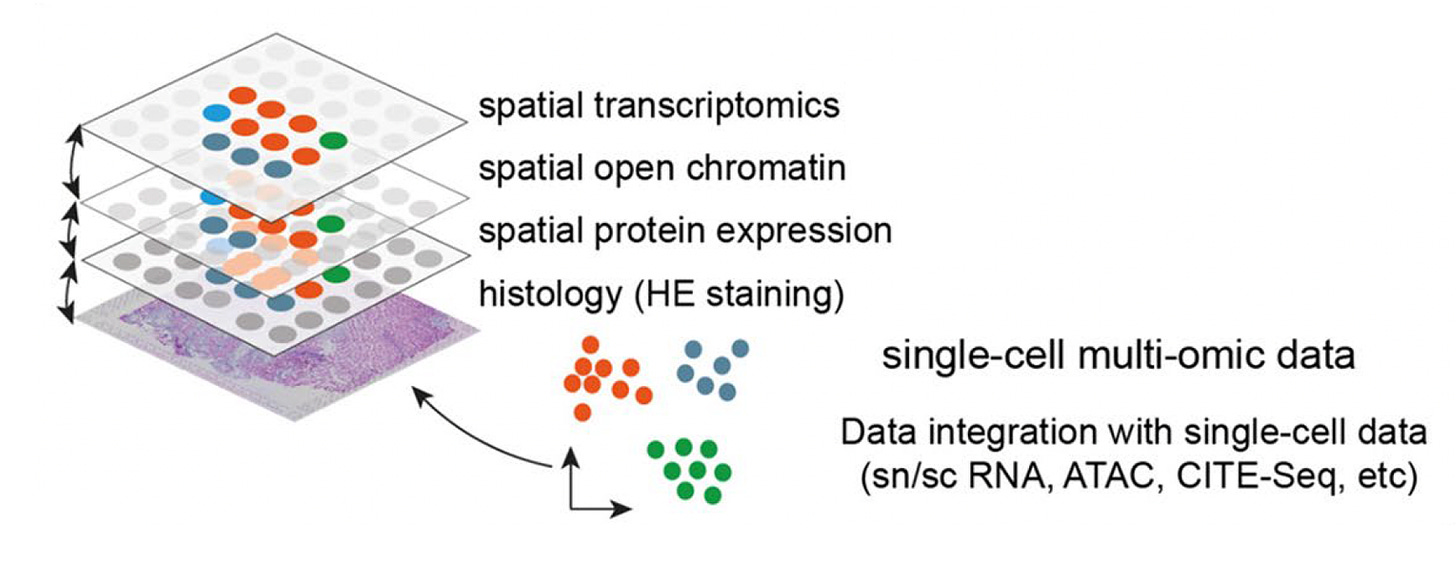
Below is a simplified graphic to show single-cell (SC, or single nuclei, SN) spatial multi-omics from a slide, with the data from each modality superimposed and integrated. Figure adopted from Kiessling and Kuppe.
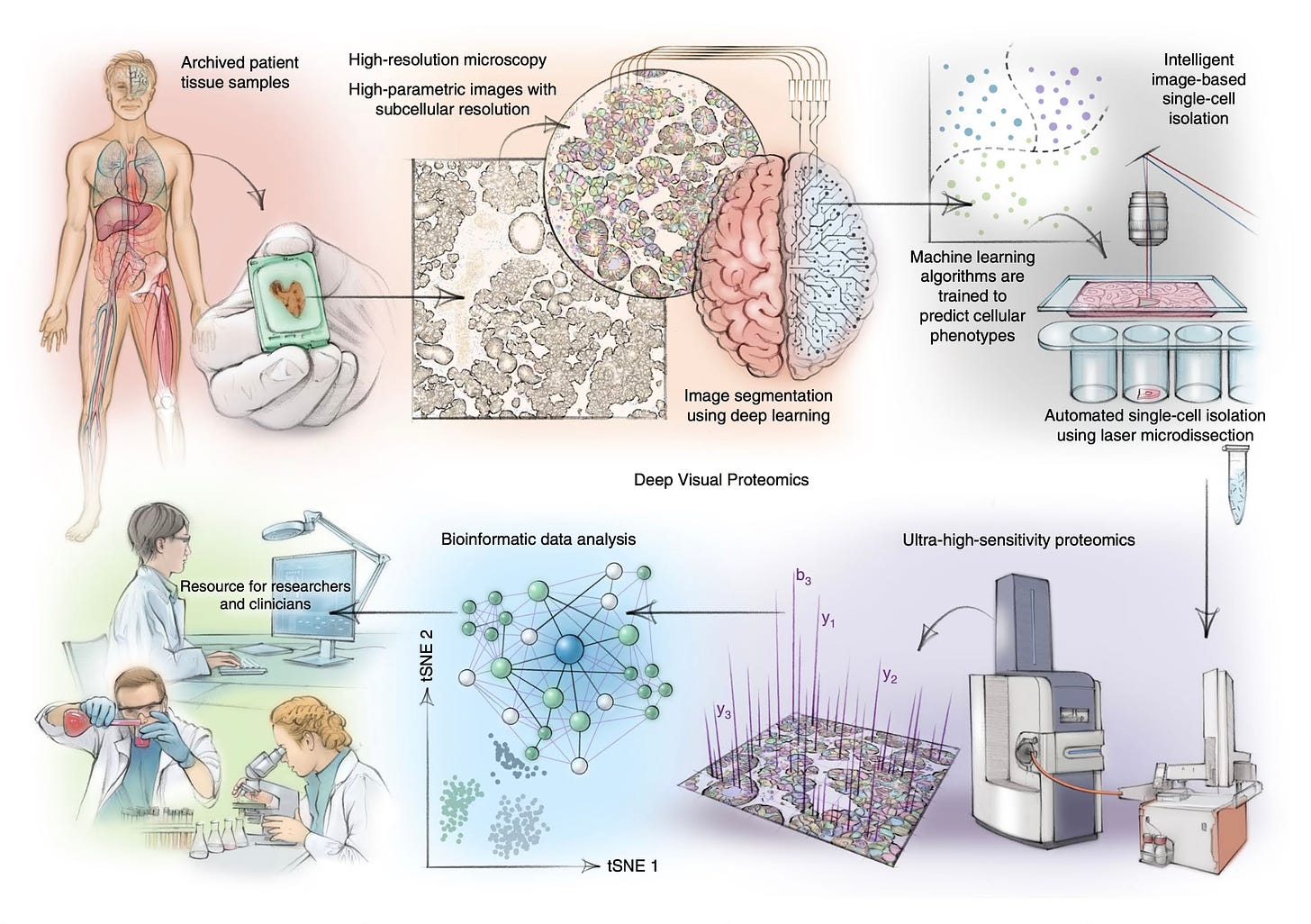
Deep Visual Proteomics (DVP)
Back in 2022, Matthias Mann and colleagues introduced what they called Deep Visual Proteomics (DVP) graphically summarized below. From a patient sample of tissue (PPFE, formalin-fixed, paraffin-embedded), advanced microscopy using deep learning A.I. segments the image with resolution at the sub-cellular level. That identifies the target cells to be analyzed. Then laser microdissection (video below) is performed to excise single cells, followed by ultra-sensitive mass spectroscopy based proteomics.
Automated laser microdissection of single immune cells, which relies on deep learning A.I. to identify them accurately.
The Seminal Paper
You may be familiar with Stevens-Johnson Syndrome (SJS), which is related to and is the more common type in the continuum of TEN, both extremely serious but thankfully rare adverse skin conditions from commonly used medications (or arising as a sequelae of infections, such as from Mycoplasma pneumoniae in children or hepatitis A). The wife of a colleague of mine nearly died from Stevens-Johnson after 1 dose of carbamazepine. SJS affects less than 10% of the body surface area while TEN involves more than 30%; the latter carries a mortality about 1 in 3 or even as high as 1 in 2. There’s a SJS-TEN overlap categorization for in-between presentation. There is no known effective treatment for either condition, nor has the mechanism been adequately defined beyond death of keratinocytes, which comprise 90% of the epidermis skin cells, and immune cell activation.
Published in Nature, Thierry Nordmann, Matthias Mann and their international consortium, used deep visual proteomics from 3μm PPFE sections of skin biopsies in patients affected by TEN, maculopapular rash (MPR), drug reaction with eosinophilia and systemic symptoms (DRESS), and in healthy controls. The number of patients assessed for each were 6, 5, 5, and 5, respectively
Segmentation of keratinocytes and immune cells, which is performed with the help of machine learning, is shown below.
The advantage of DVP single-cell proteomics vs bulk proteomics for differentiating the principal components (PC) of the keratinocytes and immune cells is shown here. As you can see at left, the 3 different skin conditions all overlap with bulk proteomics.
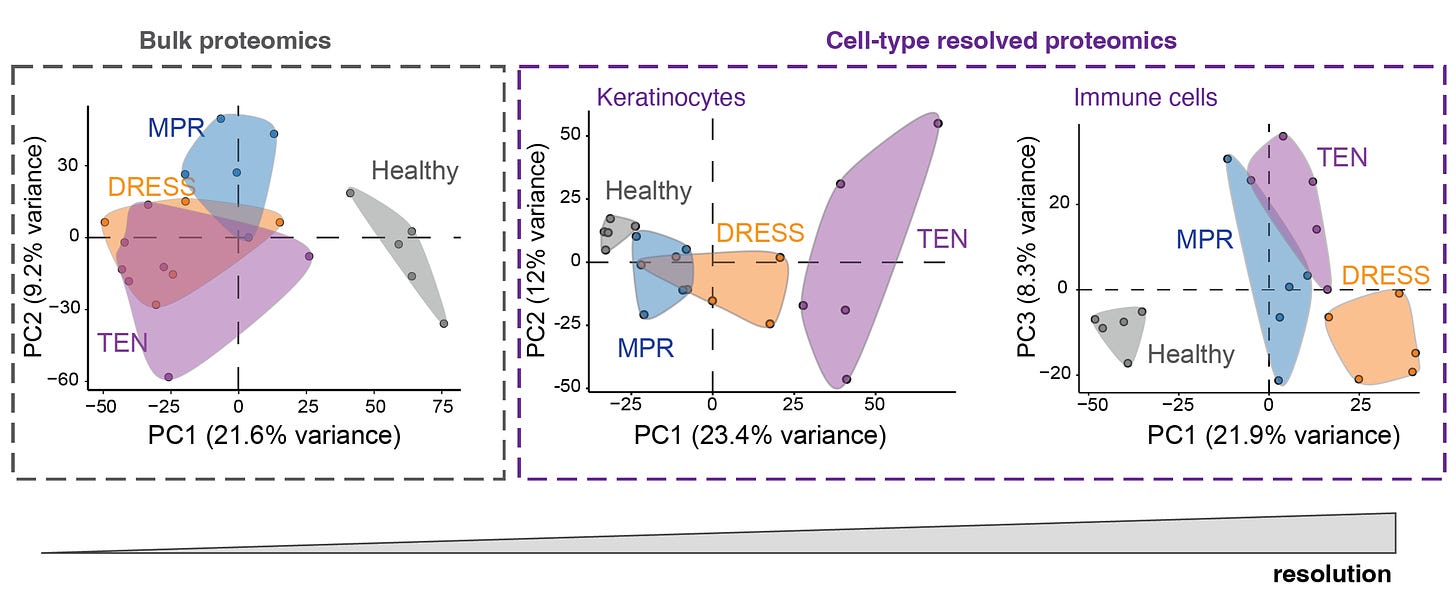
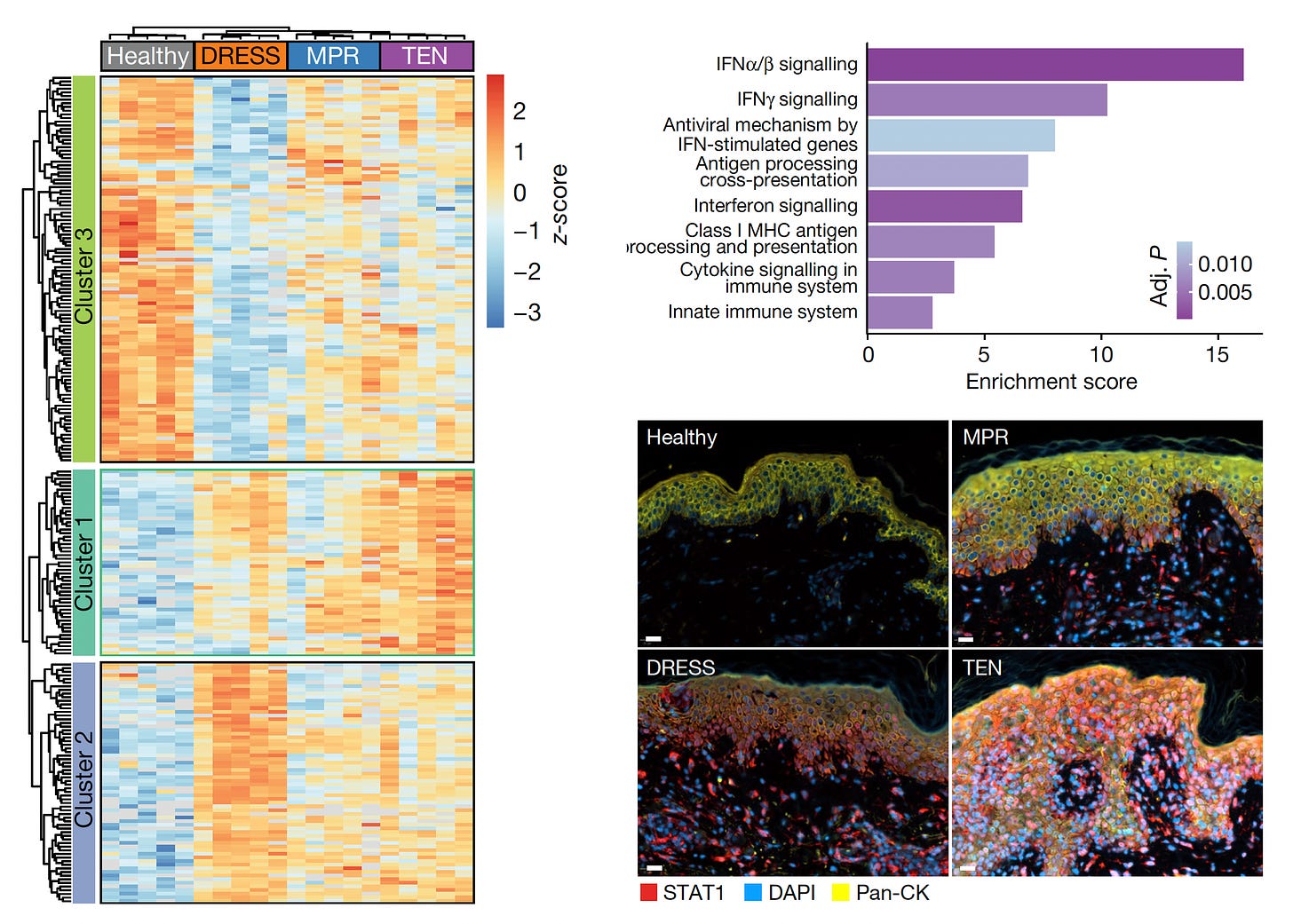
More than 5,000 proteins were quantified from single cells—keratinocyte and immune cells—using mass spec, for the 4 different skin conditions (proteome cluster in Figure below, left panel). This led to the finding that the TEN patients had marked increased in Type 1 and 2 interferon signaling and activation of phosphorylated STAT1, which invoked the janus kinase (JAK/STAT) pathway (upper and lower panels at right below). Within the immune cells, specifically the cutaneous macrophages compared with CD4+ and CD8+ T cells were noted for marked pro-infammatory activity.
Subsequent steps were to test JAK inhibitors in cell culture (with live cell imaging) and in two different mouse models, all showing highly potent, dose-dependent impact on inhibition of the intense inflammatory process and disease severity.
Most research would have finished here, providing new definitive evidence of the mechanism of TEN and potential application of JAK inhibitors in cell culture and preclinical models. That would ordinarily have been more than sufficient for a paper to be published in Nature.
Taking the Findings to Patients
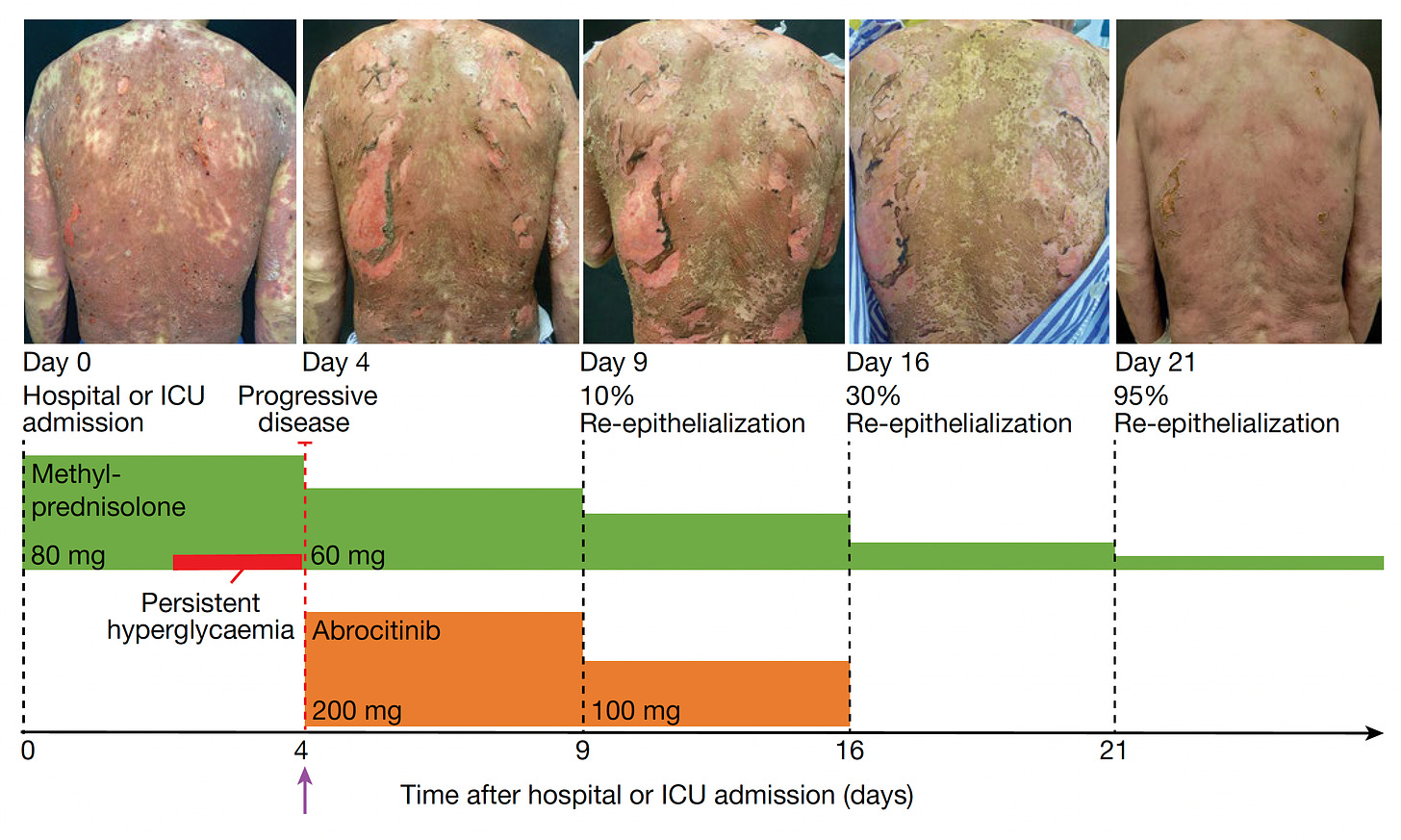
But the Mann-led international consortium work didn’t stop here! They went on to treat seven patients at Fuian Medical University, the course of one patient shown below, treated with a JAKi on day 4 after diagnosis, and manifesting a marked response starting within 48 hours. All 7 patients fully resolved, with no side effects. Unlike most first-in-human intervention reports where only the most impressive image is selected, all their photographs are presented in the supplementary information, Extended Figures 9 and 10. Warning: some of these photos are pretty gory.
Significance
This is a very impressive report, the first time we’ve seen spatial omics directly used in medicine for influencing patient care. Beyond providing mechanistic insights on the fundamental role of the JAK/STAT pathway and key cell types, a major feat on its own, the work illuminated a life-saving therapy for a disorder without a known treatment.
Demis Hassabis and John Jumpers work in developing the transformer model AlphaFold2 was recognized by the 2024 Nobel Prize in Chemistry, with prediction of 3D protein structure from amino acid sequence for about 200 million proteins. That is generally considered to be the most important impact of A.I. in life science to date. We’ve already been seeing its impact for advancing understanding of human biology and, along with protein design, accelerating drug discovery.
Spatial medicine is similar with respect to integrating biologic data with A.I., but different by directly affecting patient outcome, at least as seen in this pioneering TEN work. There will likely be many more ways that spatial medicine will move forward, such as an individualized treatment approach to a patient with cancer.
For spatial medicine, there are multiple analytical challenges that invoke the need for machine learning and A.I., including segmentation of cell types, automated capture of cells via microdissection, extracting useful information from the >5,000 proteins quantified per cell, and ultimately, as we’ll see more in the future, A.I. powering the construction of high-resolution 3D maps.
The dawn of spatial medicine coincides with current intense interest in promoting spatial intelligence, defined as the ability to understand, visualize, and interact, with our 3D world in space and time. Despite exponential growth of generative A.I., lack of its spatial intelligence is considered a major gap that holds it back. Recently, World Labs, a new start-up company led by Fei-Fei Li, has raised over $230 million of funding to develop AI systems that understand and interact with the 3D physical world.
So the interest and ability to do spatiotemporal mapping in life science and our world is gaining traction. What is exciting to see here is that it’s already unraveling the mechanistic basis of a fatal medical condition and providing a therapy that can save lives!








Post comments