by Van Andel Research Institute
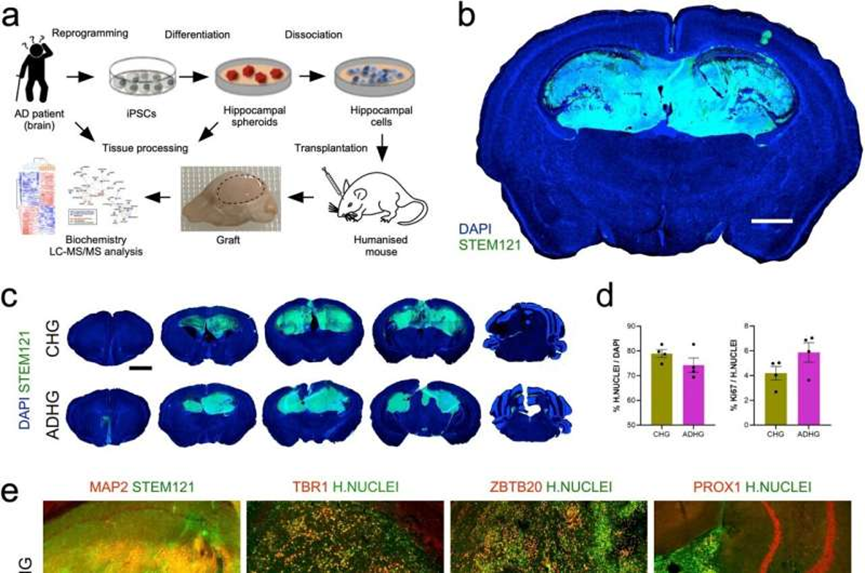
Characterization of the iPSC-derived human grafts (HG) in the mouse brain a Schematic representation of the experimental workflow. b and c Immunostaining for human cytoplasmic marker STEM121 and nuclear marker DAPI in control and AD grafts six months after transplantation into mouse hippocampus. Scale bars: 1 mm (b) and 2 mm (c). ADHG: AD human graft; CHG: Control human graft. d Quantification of human Nuclei marker-positive cells expressed relative to the total number of DAPI-labeled cells and Ki-67-positive cells expressed relative to the total number of human Nuclei marker-positive cells in control and AD iPSC-derived human grafts. Results are presented as mean ± S.E.M. N = 4 animals. Statistical analysis by two-tailed t-test. e Immunostaining for human Nuclei marker and neuronal markers MAP2, TBR1, and hippocampal markers ZBTB20 and PROX1 in control and AD iPSC-derived human grafts. Scale bars: 200 µm. f Quantification of DCX-, MAP2-, TBR1-, ZBTB20-, PROX1-, GFAP- and STEM123-positive cells in control and AD iPSC-derived human grafts. Results are presented as mean ± S.E.M. N = 4 animals. P value: ∗ p < 0.05. Statistical analysis by two-tailed t-test. Credit: Acta Neuropathologica Communications (2023). DOI: 10.1186/s40478-023-01649-z
A new model developed by Van Andel Institute, Lund University and University of Florence scientists will enable researchers to better understand how Alzheimer's disease progresses in the brain.
Like other neurodegenerative diseases, Alzheimer's is challenging to study. It is immensely complex, develops over a long period of time and varies from person to person. Critically, scientists also lack non-invasive techniques to monitor disease progression in the human brain. Instead, they often rely on models that mimic the disease, allowing them to track the ways in which Alzheimer's develops and affects the brain.
"For models to work, they must closely reflect the way Alzheimer's progresses in real life. Our new model replicates many key facets of this process," said Laurent Roybon, Ph.D., an associate professor at VAI and co-corresponding author of a study describing the model. "A strength of this work is our use of cell lines from people with Alzheimer's, which helps us better mirror the actual disease process in our model."
The study was published in the journal Acta Neuropathologica Communications. Lund University's Yuriy Pomeshchik, Ph.D., is co-corresponding author. Roybon was an associate professor at Lund University before joining VAI in 2022.
The new model leverages induced pluripotent stem cells (iPSCs), adult cells that are reprogrammed into an earlier "blank slate" state. From there, scientists can coax these cells to become other cell types, such as neurons. This powerful technique allows scientists to study human brain cells that share the same genetic background as the person from whom they are derived.
However, studying these cells outside of the brain's microenvironment presents another challenge. Brain cells benefit from a tightly regulated ecosystem that fosters cell health and function. To solve this problem, the team used iPSCs derived from people with Alzheimer's to generate brain cells.
These cells were then grafted into the brains of immunodeficient wildtype mice and monitored for the development of hallmark Alzheimer's pathologies: protein plaques and tangles, inflammation, mitochondrial dysfunction, and the ability for pathologies to move throughout the brain.
The researchers found that the model successfully replicated critical changes in brain pathways that could later lead to the formation of amyloid plaques, as well as disease-associated changes in neighboring cells, a sign that disease pathology may be spreading from cell to cell. Proteomic analysis also revealed changes that accompany progression from early to advanced stages of the disease.
The new model addresses a central problem in Alzheimer's research: Although cell and mouse models have long aided in the study of the disease, combining the two more accurately reflects how it develops and progresses in humans. Such models are called chimeric models and combine strengths of both model systems.
The next steps, Roybon said, are to monitor how cellular pathology develops over a longer timeframe and investigate how pathology differs based on the various genetic mutations that contribute to Alzheimer's. In addition, the team plans to address their new model's limitations, such as generating iPSCs that more specifically reflect cell populations in specific regions of the brain.







Post comments