by Kyoto University
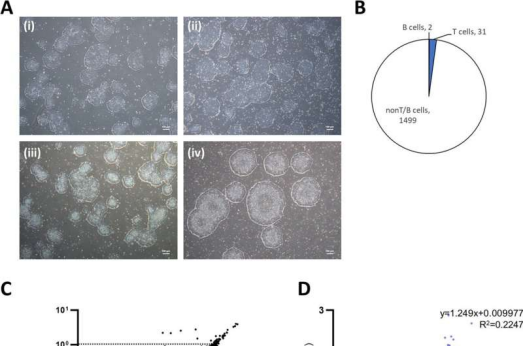
Basic characterization of established iPSCs. A Representative phase contrast images of iPSC colonies. iPSC clones from the patients with (i) Duchenne muscular dystrophy, (ii) Alexander disease, (iii) Dravet syndrome and (iv) Smith-Magenis syndrome are shown. Scale bars = 100 μm. B Estimation of the cell type from which iPSCs originated; n = 1,532. C Estimation of the number of residual copies of episomal vectors remaining in iPSCs, calculated based on the quantitative values of EBNA and CAG promotors. Dashed lines are drawn at the line corresponding to one copy of remaining vector per cell; n = 1,532. D Correlation between OCT3/4 and NANOG expression in each clone. The single regression equation is plotted on the graph; n = 1,532. E, F Comparison of OCT3/4 (E) and NANOG (F) expression by sex (female n = 798, male n = 734). Values for each clone have been plotted relative to the expression levels in control 201B7 iPSCs. Statistical analysis was performed using the unpaired t-test (E) and Mann–Whitney U test (F). Credit: Inflammation and Regeneration (2023). DOI: 10.1186/s41232-023-00294-2
A team of researchers led by Professor Megumu K. Saito have undertaken a massive effort to build an induced pluripotent stem (iPS) cell resource for rare diseases, enabling research and development of novel diagnostic tools and therapeutic treatments for 139 designated intractable diseases.
Rare diseases affect relatively few people within the general population. They are defined by the European Union and the United States as diseases affecting less than 1 in 2,000 or 1 in 200,000 people, respectively. There are currently more than 7,000 rare diseases, and patients with such diseases typically do not receive proper diagnosis or treatment. The rarity of these diseases creates hurdles to designing and conducting studies, especially large-scale controlled clinical trials, to develop diagnostic tools and therapeutic strategies.
Led by Professor Saito (Department of Clinical Application), a team of researchers recognized the grand utility of induced pluripotent stem cell (iPSC)-based disease modeling and established iPSCs from patients with designated intractable diseases in Japan.
In total, 259 patients with 139 designated intractable diseases were recruited for this effort to produce 1,532 iPSC clones. The cells were established using a standard platform and have been deposited at the RIKEN BioResource Center, available to be distributed for academic and commercial research purposes. A paper on this research was published online in Inflammation and Regeneration on September 8, 2023.
iPSC clones were established from patients with a wide range of rare diseases, including but not limited to those affecting various organ systems (e.g., nervous, circulatory, respiratory, and digestive systems) or those with congenital malformations, deformations, and chromosomal abnormalities. The researchers have made information about the donors and the iPSC clones publicly available online via the CiRA iPS Cell Line Database (CiCLeD).
Overall, iPSCs were generated from all recruited patients, with no cases of reprogramming failure. For most patients, six independent iPSC clones were generated from the blood samples of each patient. Age did not affect the reprogramming efficiency or growth rates of the iPSC lines generated. Further analyses of the generated iPSC lines revealed a weak correction between POU5F1 (OCT3/4) and NANOG expression, transcription factors expressed in undifferentiated pluripotent stem cells.
Notably, their expression levels were significantly higher in female-derived clones. The cause of this sex-dependent difference is unknown but may be of research interest because of its potential link to molecular mechanisms underlying pluripotency.
This colossal effort will hopefully jumpstart research on these rare and intractable diseases to develop diagnostic tools and treatments. It represents the first step towards true personalized medicine, not just for common diseases suffered by millions worldwide but also those affecting a relative few.







Post comments