Author links open overlay panelYa-Chieh Hsu 1, H. Amalia Pasolli 1, Elaine Fuchs
Summary
Here, we exploit the hair follicle to define the point at which stem cells (SCs) become irreversibly committed along a differentiation lineage. Employing histone and nucleotide double-pulse-chase and lineage tracing, we show that the early SC descendents en route to becoming transit-amplifying cells retain stemness and slow-cycling properties and home back to the bulge niche when hair growth stops. These become the primary SCs for the next hair cycle, whereas initial bulge SCs become reserves for injury. Proliferating descendents further en route irreversibly lose their stemness, although they retain many SC markers and survive, unlike their transit-amplifying progeny. Remarkably, these progeny also home back to the bulge. Combining purification and gene expression analysis with differential ablation and functional experiments, we define critical functions for these non-SC niche residents and unveil the intriguing concept that an irreversibly committed cell in an SC lineage can become an essential contributor to the niche microenvironment.
Graphical Abstract
Highlights
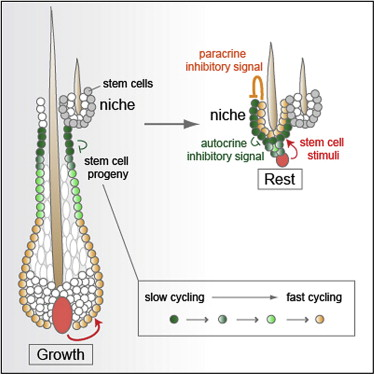
► During hair growth, early stem cell progeny transition from slow to fast cycling ► Slow-cycling progeny are the major stem cell contributors for the next hair cycle ► Fast-cycling progeny also return back to the niche but become committed ► The cells send strong inhibitory signals to balance stem cell stimuli in homeostasis
Introduction
Adult stem cells (SCs) govern tissue homeostasis and wound repair. They reside in a specific niche, defined as the microenviroment, that hosts and maintains SCs (Spradling et al., 2008). Most SCs are infrequently cycling, a feature thought to preserve their stemness, namely their ability to self-renew and remain undifferentiated over the animal's lifetime. During normal homeostasis, they often exit from their niches and progress to become transit-amplifying (TA) cells, undergoing a series of rapid divisions before committing to terminal differentiation (Fuchs, 2009, Morrison and Kimble, 2006).
Determining the point in a lineage hierarchy where SCs lose long-term self-renewing capacity and become irreversibly committed represents a fundamental and challenging question in SC biology. Transitioning from a slow-cycling to more fast-cycling state is not indicative, as hematopoietic stem cells (HSCs) and hair follicle (HF) SCs can reversibly switch from dormancy to cycling during normal homeostasis and wound repair (Blanpain et al., 2004, Foudi et al., 2009, Nowak et al., 2008, Taylor et al., 2000, Waghmare et al., 2008, Wilson et al., 2008). Merely exiting their niche is also not a reliable measure, as some HSCs circulate, trafficking between their bone marrow niche and extramedullary tissues (Cao et al., 2004). Even embarking along a differentiation pathway may not be an unequivocal indicator of loss of stemness; studies in Drosophila and mouse testis show that germline SC niche vacancies can be filled by early spermatogonial cells that dedifferentiate when returned to the niche (Brawley and Matunis, 2004, Kai and Spradling, 2004, Brinster and Avarbock, 1994).
The murine HF offers an excellent system for monitoring an SC lineage and exploring plasticity of SC progenies. During homeostasis, the lower HF cycles through bouts of active hair growth (anagen), destruction (catagen), and rest (telogen) (Lavker et al., 2003, Paus and Cotsarelis, 1999). When the new HF emerges, it grows next to the old hair, which persists into the next cycle. This creates a protrusion or “bulge,” first described >100 years ago (Unna, 1876). In 1990, nucleotide pulse-chase experiments revealed the existence of slow-cycling, label-retaining cells (LRCs) in the bulge (Cotsarelis et al., 1990). A decade later, these cells were isolated, characterized, and shown to self-renew long-term and contribute to HF lineages and wound repair (Blanpain et al., 2004, Claudinot et al., 2005, Ito et al., 2005, Morris et al., 2004, Tumbar et al., 2004, Zhang et al., 2009). These findings established the bulge as a bona fide HF-SC niche.
Hair growth is fueled by bulge SCs, which are activated at the start of anagen by the dermal papilla (DP), a cluster of underlying mesenchymal cells. Upon activation, SCs exit the bulge and proliferate downward, creating a long linear trail of cells, the outer root sheath (ORS) (Ito et al., 2005, Zhang et al., 2009). In mature HFs, the ORS extends from bulge to matrix. Enveloping the DP at the HF base, matrix cells cycle rapidly but transiently before differentiating upward to generate the hair and its channel (Figure 1A and Figure S1A available online).
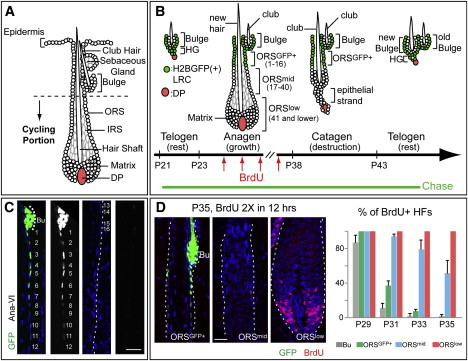
Figure 1. Dynamics of Slow- and Fast-Cycling Cells throughout the Hair Cycle
(A) Cycling portions of a mature HF. (B–D) Tet-Off H2BGFP mice were chased from P21 to the days/stages noted (P35–P37 corresponds to AnaVI). Before harvesting skin, some mice were given 2×6 hr pulses of BrdU. Schematic depicts H2BGFP cells (green) that will be label-retaining (LRCs) when the chase is stopped at each stage. Cell #s are counting down from the bulge base (= 0). In (C), the right panels of each pair are duplicates of GFP monochromes of the left panels. Scale bars, 30 μm. Bu, bulge. DAPI in blue. In (D), note that S phase cells in AnaVI are mainly in ORSlow and matrix. %HFs with BrdU cells in bulge or in different ORS segments were quantified from P29–P35. For each stage, n = 2 mice and ≥23 HFs/mouse were counted. Data are mean ± standard deviation SD.+
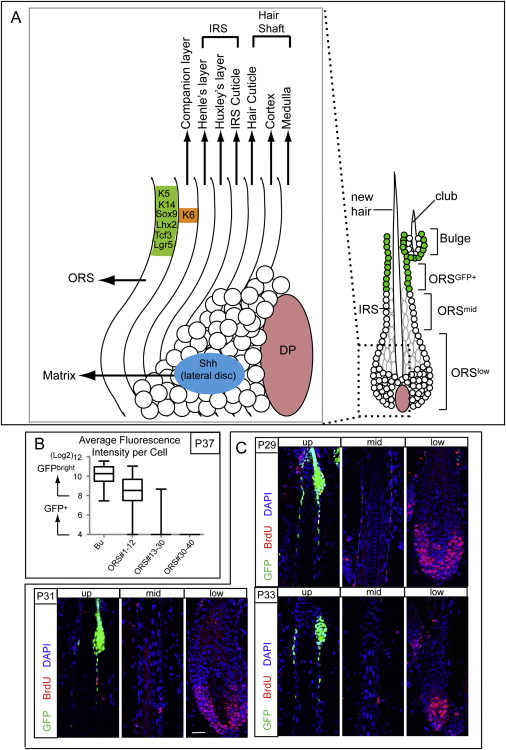
Figure S1. Schematic of an Anagen Hair Follicle, and Proliferation Analyses of Anagen Outer Root Sheath Cells, Related to Figure 1 and Figure 4
(A) Schematic. During anagen, matrix cells (also referred to as transit-amplifying or TA cells) are located at the base of the growing follicle. There, they form a bulb that envelops the dermal papilla (DP). Mesenchymal-epithelial interactions maintain matrix cells in a rapidly proliferative and relatively undifferentiated state, but also provide fate-instructive properties. As matrix cells move upward, they terminally differentiate into one of the seven distinct lineages that are spatially organized into concentric rings in an onion-like fashion: the medulla, cortex and cuticle of the hair shaft; the cuticle, Henle and Huxley layers of the inner root sheath (IRS), and the companion layer (Cp), which is sandwiched between the IRS and ORS. Shh, Sonic hedgehog, a marker of the lateral disc, which is a small pocket of cells that gives rise to the IRS layers. K6 is a marker of the anagen-phase companion layer. Sox9, Lhx2, Tcf3, and Lgr5 are all markers that are enriched in the CD34 bulge cells, but also expressed throughout the ORS cells between the bulge and the matrix (Greco et al., 2009). K5 and K14 are markers of the basal cells of the epidermis, ORS, sebaceous gland and bulge.+
(B and C) These data pertain to Figure 1 and Figure 4. (B) shows the average epifluorescence intensity of individual cells in the bulge (Bu) and ORS of Tet-OFF H2BGFP HFs, which were chased beginning at P21 at the start of the first telogen phase of the hair cycle and then analyzed at P37, the end of the anagen phase. The data are plotted as box-and-whisker diagram, indicating the median (middle line), 25–75th percentile (box), minimum and maximum (whiskers), n = 22 HFs. Note that at this time, the upper ORS cells (positions 1–12) display slow-cycling characteristics similar to the initial bulge, but they have undergone on average 1-2X more divisions, reflected in their reduced epifluorescence. Cells below position 30 divided more times, and GFP levels dipped below detection limits. (C) Examples of Tet-OFF H2BGFP HFs chased since P21 and then analyzed at P29–P33. Just prior to analyses, mice were given two short (6 hr) BrdU pulses so that we could track the proliferative behavior of all the HF cells at this time (BrdU) and contrast this with their slow cycling nature (GFP). Note that at P29 mid-anagen, there are many double positive cells in the bulge and in the ORS++GFP+ zone. At P31, proliferation in the bulge has ceased while proliferation events are still detectable throughout the ORS. By P33, the upper ORS has mostly withdrawn from the cell cycle. By contrast, the lower ORS and matrix are hyperproliferative throughout this period.
Catagen illuminates an unambiguous distinction between long-lived HF-SCs and short-lived matrix progeny that undergo massive apoptosis. The remaining epithelial strand retracts, drawing the DP upward. Current evidence suggests that at the catagen/telogen transition, a few bulge SCs migrate to meet the DP, generating the hair germ (HG) (Ito et al., 2004, Zhang et al., 2009). Bearing closer resemblance to bulge than matrix, HG cells are activated prior to bulge at the start of anagen (Greco et al., 2009). Prior to activation, HF-SCs undergo an extended rest period that can last for months.
Whereas extensive studies have been performed on bulge SCs and TA-matrix cells, the properties and fates of ORS cells are less clear. Although these cells do not express high levels of CD34, a marker of their SC bulge predecessors, they display many HF-SC markers not found in matrix, including Lgr5, Sox9, Lhx2, and TCF3 (Fuchs, 2009). To date, functional studies on ORS cells have been limited to cultures of microdissected rat whisker HFs, where long-term clones capable of engraftment were obtained not only from bulge and ORS but also from matrix (Claudinot et al., 2005, Oshima et al., 2001, Rochat et al., 1994). Whether ORS cells in vivo possess stemness like their predecessors or are committed like their TA progeny remains unknown.
The unique regenerative aspects of HF homeostasis and the transient, but spatially and temporally well-defined ORS offer an unparalleled opportunity to examine the transition between a SC and a TA cell. Where does the transition from slow cycling to fast cycling occur along the ORS trail? What happens to ORS cells during catagen? Do they undergo apoptosis like TA cells, or do they survive like SCs? If they survive, do they home back to the bulge, and if they do so, do they function as SCs?
Answering these questions could provide fundamental insights into defining not only the importance of bulge to SC survival but also the point where an SC loses its stemness and embarks upon a terminal differentiation pathway. If all ORS cells either die or differentiate during catagen, this would imply that HF-SCs lose stemness once they leave the bulge. If on the other hand, some ORS cells survive and return back to the bulge as functional SCs, this would imply that at least at some point along the progression from bulge to TA compartments, an SC that has left the bulge still possesses intrinsic features of stemness.
In this report, we address these key questions in the unperturbed in vivo confines of the normal hair cycle, as well as in response to injury. In so doing, we unearth surprising and dynamic features of the HF-SC niche and its progeny. First, we show that during anagen, the upper ORS cells remain slow cycling, like their SC predecessors, whereas lower ORS cells cycle more rapidly. Moreover, slow-cycling ORS cells survive catagen and contribute mightily to both HG and a new bulge: in fact, they become the main source of SCs used during the next hair cycle. Lacking a DP, the initial bulge ceases to play a major role in homeostasis but can respond to injury. Finally and perhaps most surprisingly, some actively cycling lower ORS cells not only survive catagen but also home back to the bulge. Like their upper ORS predecessors, these cells retain many HF-SC markers. However, they irreversibly lose their ability to proliferate in normal homeostasis or upon wounding. Instead, they function decisively in hair anchorage during the resting phase and in providing the quiescent signaling cues that control the hair cycle. Our findings define a point along the ORS where cells lose stemness and become irreversibly fated to differentiate or die and illuminate how downstream SC progeny can provide negative feedback to the niche and restrict SC self-renewal and tissue formation.
Results
Bulge Descendents in Anagen ORS Can Be Subdivided into Slow Cycling and Faster Cycling
To dissect the properties of bulge SC descendents along the ORS, we first addressed whether these cells remain slow-cycling like bulge or become faster-cycling like matrix. We used bi-transgenic mice expressing Doxycycline (Doxy)-repressible histone H2BGFP controlled by a keratin 5 (K5) promoter. With this Tet-Off system, skin epithelium is uniformly labeled until Doxy exposure, when new H2BGFP synthesis is tightly repressed and cells deplete existing GFP by 2×/division (Tumbar et al., 2004).
Doxy chase was begun at postnatal day (P)21, just before the start of the 1st hair cycle (Figure 1B). At the last stage of anagen (AnaVI, based on Muller-Rover et al., 2001; P35–P37 in these mice), the bulge but not matrix contained bright H2BGFP LRCs (Figures 1C and 1D). Interestingly, an LRC trail extended from the bulge base along the ORS. To quantify, we standardized image acquisition for GFP intensities by setting the detector so that brightest bulge cells were maximal but not saturated in a 12 bit (= 4096 shades) image. Under this condition, bulge was composed almost exclusively of cells with GFP intensities >256 (GFPbright). Within the first 16 positions counting down along the ORS trail in a planar section, >85% of cells were GFP (intensity > 16) with the majority of GFP+bright cells in #1–12. By contrast, few ORS cells in sites 16–30 were GFP, and below #30, GFP was not detected (Figure 1C and Figure S1B).+
To further probe proliferation heterogeneities during anagen, we performed a series of short BrdU pulse experiments in Tet-Off H2BGFP mice. When labelings were conducted at intervals between P27 and P29, i.e., after HFs displayed an emerging inner root sheath (IRS) and typical anagen shape, BrdU cells were detected in bulge, ORS, and matrix. By P31, however, most bulge cells had ceased proliferation. Although occasional BrdU cells were still seen in ORS positions 1–16, proliferation was mostly beneath this zone in ORS and matrix. By P33, most BrdU ORS cells were below #30, and from P35–P37, BrdU cells were largely restricted to matrix, with few labeled ORS cells <#40 (Figure 1D and Figure S1C). The transition between ORS and matrix was at ∼#75–85.++++
To summarize: ORSGFP+(#1–16) behaved similarly to bulge, displaying slow-cycling characteristics and returning to quiescence ∼2 days after bulge proliferation ceased; ORSmid (#17–40) remained proliferative 2 days after ORSGFP+; and ORSlow cells (#40–80) and matrix cycled continuously.
SCs that Exit the Bulge during the Growth Phase and Remain Slow Cycling (ORSGFP+) Are Spared From Apoptosis during the Destructive Phase
Because ORSGFP+ cells divide only a few times during anagen, we next wondered what happens to these cells during the destructive phase. Throughout catagen, the ORSGFP+ trail showed no signs of migration or expansion (Figure 2A). Three possible lineage behaviors could explain this result: (1) GFP ORS cells are static in catagen; (2) the influx of bulge cells to ORS+GFP+ is compensated by an efflux of ORSGFP+ cells, proliferating and moving toward matrix; (3) ORSGFP+ cells undergo apoptosis and bulge cells migrate downward to replace them.








Post comments