by Justin Jackson , Medical Xpress
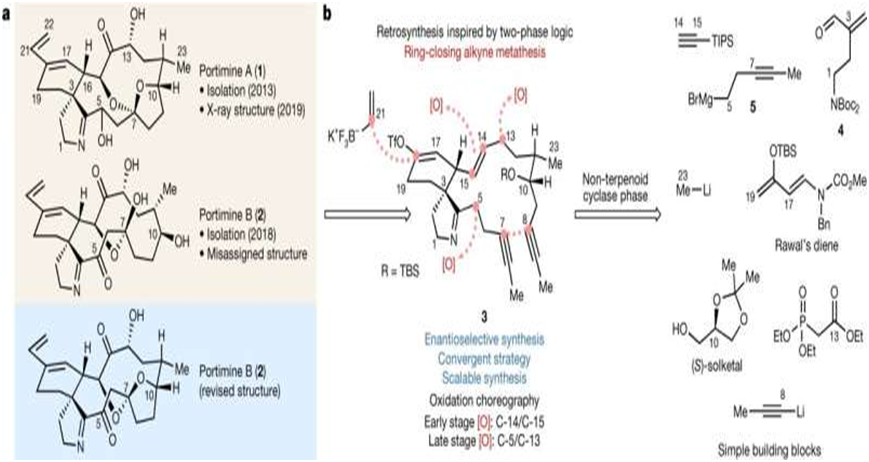 a, Chemical structure of reported PA and PB (in beige), and the revised structure of PB (in blue) in this work. b, A retrosynthesis targeting a minimally oxidized intermediate (3) leads to simple commercially available building blocks, which mimics terpenoid two-phase synthesis. Credit: Nature (2023). DOI: 10.1038/s41586-023-
a, Chemical structure of reported PA and PB (in beige), and the revised structure of PB (in blue) in this work. b, A retrosynthesis targeting a minimally oxidized intermediate (3) leads to simple commercially available building blocks, which mimics terpenoid two-phase synthesis. Credit: Nature (2023). DOI: 10.1038/s41586-023-
Researchers at the Department of Chemistry, Scripps Research, California, have discovered how to harness the toxic power of plankton to manufacture anti-cancer molecules.
In a paper, "Synthesis of portimines reveals the basis of their anti-cancer activity," published in Nature, the team details the steps taken in synthesizing marine toxins, portimine A and portimine B, enabling in-depth investigations into their properties.
Dinoflagellate-derived cyclic imine toxins, specifically portimine A and portimine B, are of interest due to their potential anti-cancer therapeutic properties. Previous research has shown the effects of cyclic imine toxins on cancer cells, but the molecular mechanisms underlying the cause of the anti-cancer activity were unknown.
Access to these toxins in large quantities is currently hard to come by as the only known producer is a type of tiny marine plankton, Vulcanodiniumrugosum. To test the toxin's activity, the researchers first needed to innovate a way to synthesize large enough quantities to work with.
The synthesis began with constructing a minimally-decorated carbon skeleton devoid of most oxygen atoms. The idea was to leverage a macrocycle's innate reactivity to install the correct oxygenation pattern and stereochemistry.
Strategic ring-chain tautomerization events were employed to facilitate the synthesis using ring-closing alkyne metathesis to construct the 14-membered macrocycle in the portimines' skeleton. The innovation represents a scalable and concise synthesis of portimines. With the desired molecules created, the next step was to see how they interacted with cancer cells.
The team tested interactions across a broad panel of 20 human and mouse cancer cell lines, ranging from Jurkat leukemia to metastatic human fibrosarcoma cells, triple-negative breast cancer and glioblastoma brain-tumor-initiating cell lines. Consistent potent cytotoxic activity was observed across the entire panel of cancer cell lines that were evaluated with portimine A. The fully synthetic portimine B was found to be substantially less effective.
Portimine A was identified as a potent inducer of apoptosis in various cancer cell lines, including MC38 cells, a colorectal carcinoma testing model. The apoptosis caused by portimine A had minimal effects on non-cancerous cells and low toxicity in mice.
Specifically, portimine A was found to target the 60S ribosomal export protein NMD3, blocking polysome formation and inhibiting protein translation and was observed to be an effective agent for suppressing tumor growth in vivo.
The exposure time to portimine A was limited by its half-life of around 30 minutes. The short duration still resulted in a significant reduction in tumor growth, indicating a very potent activity and potential therapeutic uses in the future.
Proteomics experiments revealed that the ribosomal export protein NMD3 is the target of, and necessary for, portimine A's cytotoxic activity. NMD3 is involved in ribosome assembly.
Portimine A was acting to stabilize NMD3 in a dose-dependent way, leading to decreased levels of cancer-specific proteins, MYC and MCL-1. This decrease occurred without affecting MYC and MCL1 mRNA levels, suggesting that PA inhibits the translation, not transcription, of these proteins.
As with any good research, the results indicate that more research is needed. The authors suggest that these future investigations should assess whether malignancies of certain dysregulated gene expressions are more vulnerable to portimine A and whether there are synergistic therapy applications as observed with other translation inhibitors.








Post comments