Targeting amino acid metabolism to alleviate vascular stiffening and improve pulmonary hypertension
Credit:Dietary intake and glutamine-serine metabolism control pathologic vascular stiffness
Pulmonary hypertension (PH) is a devastating condition characterized by elevated blood pressure in the pulmonary arteries, leading to increased workload on the right ventricle and eventual heart failure. The pathogenesis of PH is complex and multifactorial, involving the activation and proliferation of vascular fibroblasts, which in turn contribute to the excessive deposition of collagen and subsequent stiffening of the vessel wall. This stiffening leads to increased resistance to blood flow, further exacerbating the disease and compromising pulmonary function.
A recent research article delved into the intricate relationship between metabolism and pathologic vascular stiffness, particularly in the context of pulmonary hypertension (PH). The authors, led by Nesrine S. Rachedi and Ying Tang, employed a multifaceted approach, combining metabolomics, genetic manipulation, pharmacological intervention, and dietary modification, to uncover the metabolic rewiring of activated vascular fibroblasts (PAAFs) and its impact on collagen biosynthesis and vascular stiffness.
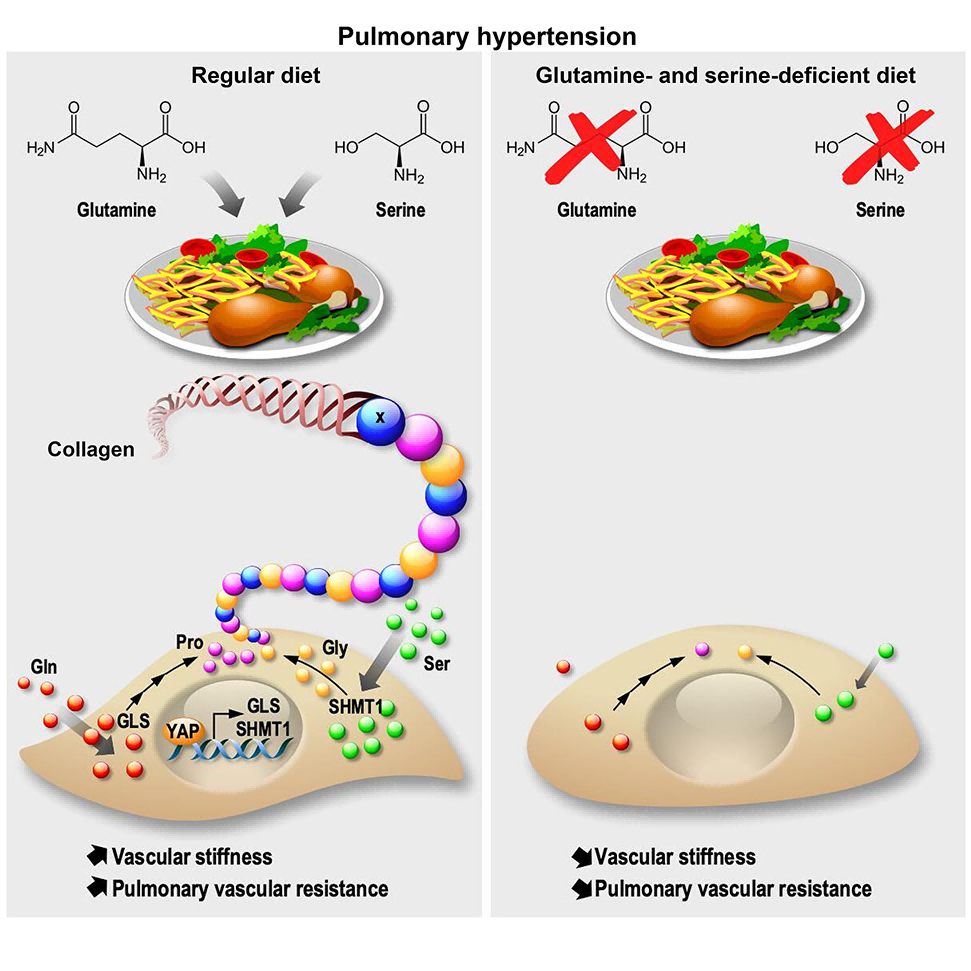
The study identified the transcriptional coactivators YAP and TAZ as central regulators of PAAF activation. These factors were activated by inflammatory, hypoxic, and mechanical stress signals commonly encountered in PH. Once activated, YAP and TAZ orchestrated a complex metabolic shift within PAAFs, favoring the catabolism of glutamine and serine to produce proline and glycine. These amino acids were essential building blocks for collagen synthesis, which in turn contributed to the pathologic stiffening of blood vessels.
The researchers demonstrated that activation of PAAFs led to a significant upregulation of glutamine and serine uptake, coupled with increased catabolism of these amino acids. This metabolic reprogramming allowed PAAFs to efficiently produce proline and glycine, which were crucial for collagen synthesis and the subsequent development of a pro-proliferative vascular niche. This niche promoted the proliferation of other vascular cells, further exacerbating the stiffening and remodeling of the vessel wall.
To further elucidate the role of YAP/TAZ in collagen metabolism, the authors investigated the binding sites of the transcriptional enhancer associated domains (TEADs), which YAP and TAZ interacted with. They found that TEADs regulated a broad spectrum of genes involved in glutamine/proline and serine/glycine metabolism, as well as collagen metabolism. This suggested that YAP/TAZ directly controlled the transcription of these genes, thereby orchestrating the metabolic rewiring necessary for collagen synthesis.
To investigate the in vivo impact of YAP/TAZ and glutamine/serine metabolism on collagen synthesis and PH progression, the authors employed genetic deletion and pharmacological inhibition strategies. Conditional knockout of YAP and TAZ in the pulmonary arteries of mice with IL-6-driven PH led to a significant reduction in collagen content, decreased vascular stiffness, and improved PH symptoms. Similarly, pharmacological inhibition of glutaminase (GLS1) and serine hydroxymethyltransferase (SHMT1) in rats with monocrotaline-induced PH results in decreased collagen production, reduced vascular stiffness, and improved pulmonary function.
Building on their findings, the authors explored the potential of dietary intervention as a novel therapeutic approach for PH. They demonstrated that a diet low in glutamine and serine effectively reduced plasma levels of these amino acids and decreased collagen content in the lungs of rats with PH. This dietary modification also led to reduced vascular stiffness and improved PH symptoms.
This study provided a comprehensive understanding of the metabolic pathways involved in the pathogenesis of PH and offered promising avenues for therapeutic intervention. The identification of YAP/TAZ as key regulators of collagen metabolism opened the door for targeted pharmacological therapies, potentially in combination with GLS1 and SHMT1 inhibitors. Furthermore, the findings highlighted the potential of dietary modification as a non-invasive and potentially effective strategy for managing PH and other fibrotic diseases.
The study acknowledged several limitations, including the potential contribution of smooth muscle cells to the observed phenotypes and the need for further investigation of the dynamic and bidirectional contributions of metabolites across different compartments. Future research should also explore the potential interplay between glutamine/serine metabolism and other metabolic pathways, as well as the long-term effects of dietary interventions.
Overall, this research provides a compelling framework for understanding the metabolic basis of pathologic vascular stiffness and offers novel insights into the potential for targeted metabolic therapies for PH and other fibrotic diseases.
Reference:
Rachedi NS, Tang Y, Tai YY, Zhao J, Chauvet C, Grynblat J, Akoumia KKF, Estephan L, Torrino S, Sbai C, Ait-Mouffok A, Latoche JD, Al Aaraj Y, Brau F, Abélanet S, Clavel S, Zhang Y, Guillermier C, Kumar NVG, Tavakoli S, Mercier O, Risbano MG, Yao ZK, Yang G, Ouerfelli O, Lewis JS, Montani D, Humbert M, Steinhauser ML, Anderson CJ, Oldham WM, Perros F, Bertero T, Chan SY. Dietary intake and glutamine-serine metabolism control pathologic vascular stiffness. Cell Metab. 2024 Jun 4;36(6):1335-1350.e8.




Post comments