Among the cardiovascular diseases, myocardial infarction (MI) is a well-deserved “health killer”, and its morbidity and mortality rates are increasing year by year, seriously threatening human life and health. When MI occurs, local ischemia and hypoxia will lead to a large number of functional cardiomyocyte necrosis and apoptosis, and vascular blockage will hinder the transportation of nutrients needed for repair of damaged myocardium, which will ultimately lead to ventricular remodeling, decline in cardiac function, and even the development of heart failure. Although current interventional, surgical, and pharmacologic treatments can improve cardiac function to a certain extent, none of them can replenish the lack of cardiomyocytes caused by infarction. Therefore, the search for therapeutic strategies to promote cardiac regeneration is urgent.
It was found that adult mammalian cardiomyocytes have limited ability to self-repair after myocardial injury, whereas the neonatal mammalian heart has a strong regenerative capacity within the first 7 days of life, and is able to replenish the damaged cells through the division and proliferation of cardiomyocytes. This finding brings new ideas for adult MI treatment. Extracellular vesicles (EVs), as important carriers of intercellular communication, show great potential in the field of cardiovascular disease treatment.
A recent study published in Nat Commun Cardiac repair using regenerating neonatal heart tissue-derived extracellular vesicles focuses on neonatal heart tissue-derived EVs and opens up a new direction for the treatment of myocardial infarction.

The researchers extracted EVs from neonatal mouse heart tissues (Neo-EVs), neonatal heart tissues regenerated after apicoectomy (AR-Neo-EVs), and adult mouse heart tissues (Adu-EVs.) The experiments demonstrated that the AR-Neo-EVs excelled in promoting cardiomyocyte proliferation, anti-apoptosis, and angiogenesis.
In in vitro experiments, Neo-EVs and AR-Neo-EVs were able to dose-dependently increase the number of Ki67- and EdU-positive cardiomyocytes and AR-Neo-EVs were more capable of promoting cell cycle re-entry compared with control and Adu-EVs. Meanwhile, AR-Neo-EVs and Neo-EVs significantly reduced apoptosis in cardiomyocytes after hypoxia, whereas Adu-EVs had no such effect.
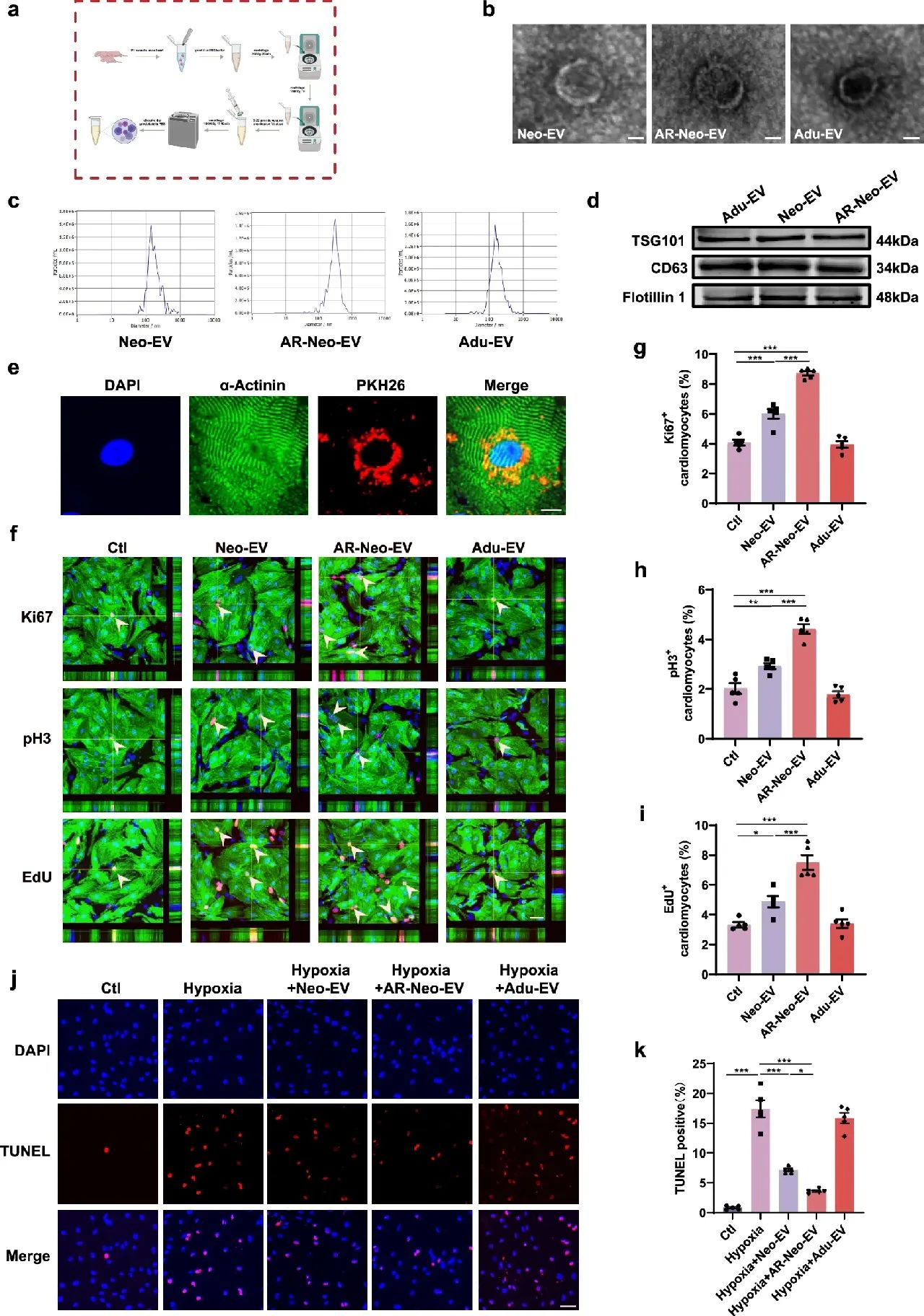
Figure 1: Extracellular vesicles from neonatal mouse heart tissue (Neo-EVs), regenerated neonatal heart tissue from transapical resection (AR) (AR-Neo-EVs), and extracellular vesicles of cardiac origin from adult mice (Adu-EVs), and their effects on cardiomyocyte proliferation and apoptosis in vitro
In the angiogenesis assay, AR-Neo-EVs also performed well. Compared with Neo-EVs, human umbilical vein endothelial cells (HUVECs) treated with AR-Neo-EVs formed more tubular networks and promoted endothelial cell migration better, while Adu-EVs failed to enhance angiogenesis.
In vivo experiments further validated the advantages of AR-Neo-EVs. The researchers established an adult mouse MI model and injected different sources of EVs in the infarct border region.The results showed that Neo-EVs and AR-Neo-EVs treatments significantly improved the left ventricular ejection fraction (EF) and shortening fraction (FS) in mice, and AR-Neo-EVs had a more pronounced protective effect on cardiac function, whereas there was no significant difference between the Adu-EVs-treated group and the MI group in terms of cardiac function. There was no significant difference. In addition, AR-Neo-EVs treatment reduced the area of myocardial fibrosis, increased the number of proliferating cardiomyocytes in the infarct border region, and decreased the number of apoptotic cardiomyocytes, while promoting angiogenesis.
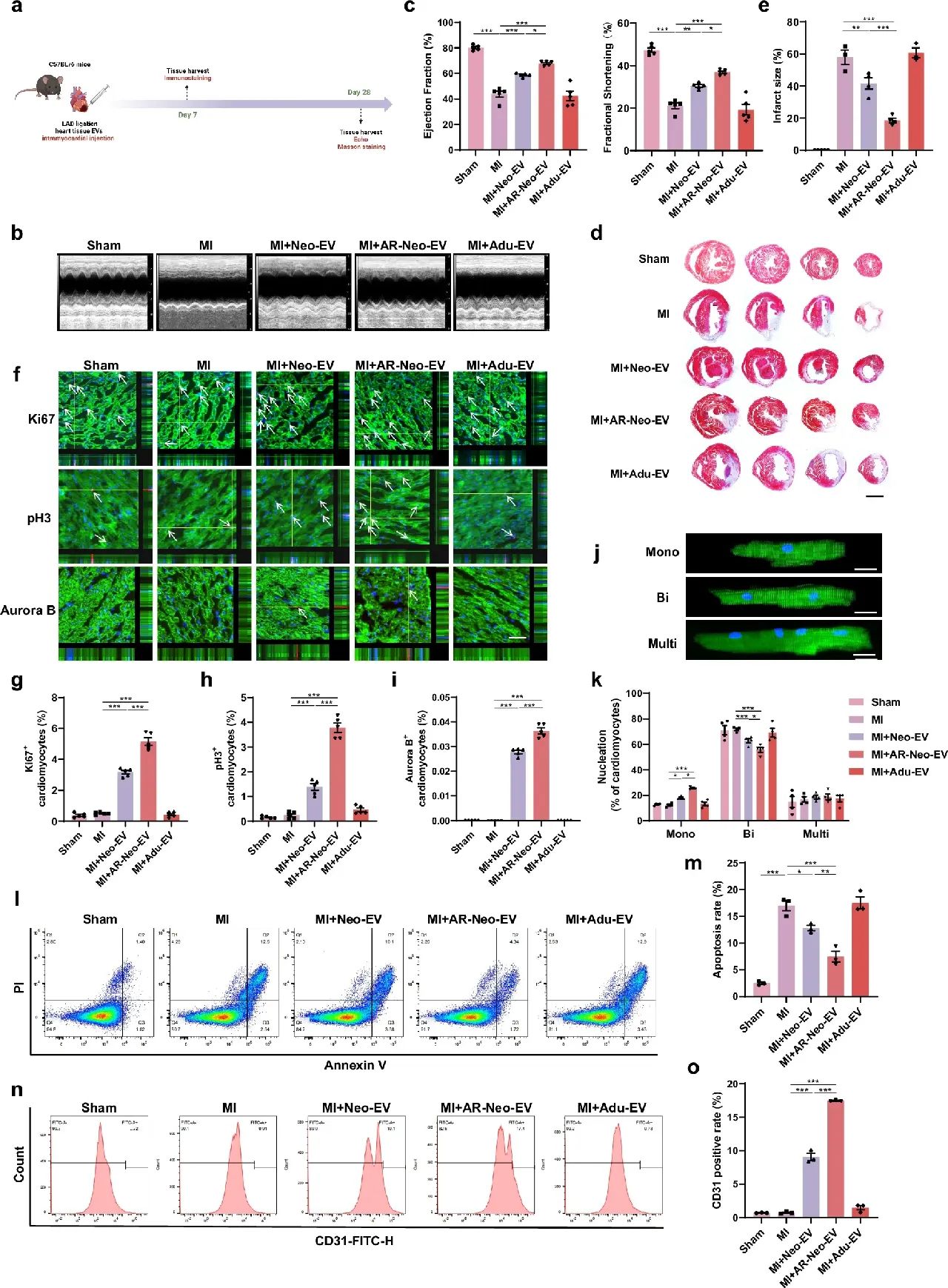
Figure 2: AR-Neo-EVs promote cardiac repair after myocardial infarction in adult mice
To address the short half-life and low tissue retention of EVs in vivo, researchers prepared sodium alginate hydrogel microspheres (MS) encapsulating AR-Neo-EVs. Experiments showed that MS could continuously release AR-Neo-EVs for up to 14 days. Compared with direct injection of AR-Neo-EVs, AR-Neo-EVs delivered via MS were more effective in improving cardiac function, reducing myocardial fibrosis, and further promoting cardiomyocyte proliferation, inhibiting apoptosis, and promoting angiogenesis in mice.
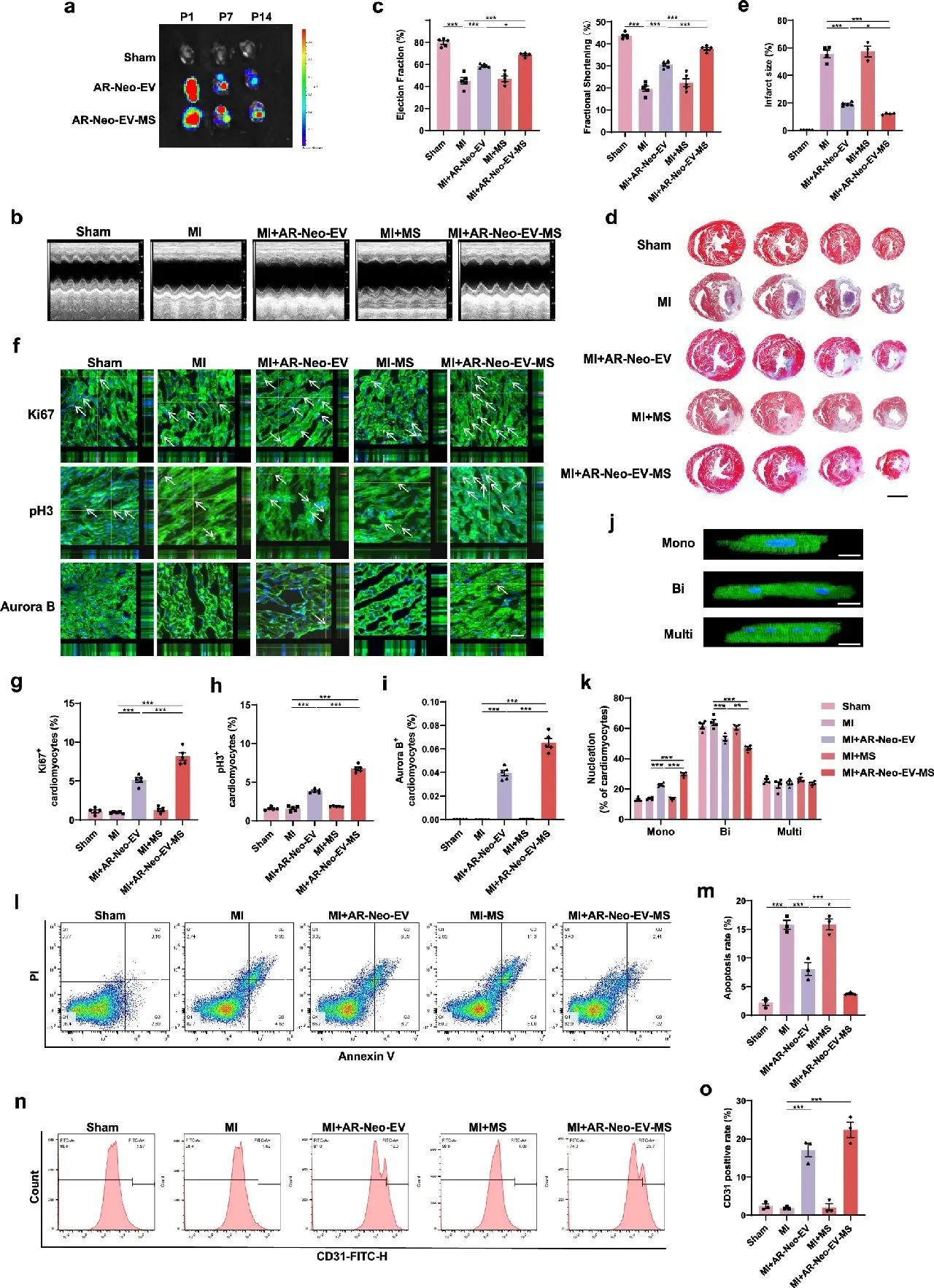
Figure 3: Delivery of AR-Neo-EVs by MS further promotes recovery of cardiac function after myocardial infarction
In-depth proteomic analysis revealed that Wdr75 protein was highly expressed in AR-Neo-EVs and played a critical role in mediating cardioprotective effects. The ability of AR-Neo-EVs to promote cardiomyocyte proliferation, anti-apoptosis and angiogenesis was significantly attenuated by knocking down Wdr75. Further studies confirmed that Wdr75 acted by regulating the p53-p21 signaling pathway.
This study demonstrated that AR-Neo-EVs have strong cardiac regenerative ability and are expected to be an effective treatment for ischemic heart disease. The application of sodium alginate hydrogel microsphere delivery system provides even stronger support for its clinical translation. However, the study is still in the basic experimental stage, and there is still a long way to go before clinical application.
In the future, more research is needed to further clarify its mechanism of action, optimize the delivery system, and evaluate long-term safety and efficacy. But no matter what, this research result brings new hope for the treatment of myocardial infarction and gives us confidence in conquering cardiovascular diseases.





Post comments