In the realm of cancer research, a groundbreaking discovery published in the prestigious journal Nature has unveiled a startling truth about small cell lung cancer (SCLC), one of the most aggressive forms of malignancy. This finding not only deepens our understanding of the disease but also holds the potential to revolutionize future treatment strategies.
SCLC is notorious for its rapid tumor growth and high propensity for metastasis. Alarmingly, two - thirds of patients are already grappling with the most perilous aspect of cancer - metastasis - at the time of initial diagnosis. However, a recent study by researchers at the Francis Crick Institute has shed light on the underlying mechanism that makes SCLC so aggressive.
The study reveals that cancer cells in SCLC possess an extraordinary ability - they can generate electrical discharges. Using advanced neuroscience techniques like patch - clamp electrophysiological recordings, the researchers found that a significant portion of cancer cells within SCLC tumors exhibit electrical excitability similar to that of neurons. This includes processes such as membrane depolarization, specific ion flow patterns, and the generation of action potentials. Complementing these "neuron - like" cancer cells, another subset within the tumor behaves much like astrocytes in the nervous system. These cells support the discharging cancer cells by supplying them with nutrients and energy, effectively creating an independent "electrical grid" within the tumor. This self - constructed electrical network drives an autonomous cycle that fuels tumor development and metastasis.
This isn't the first time cancer cells have shown a knack for mimicking neural cells. Previous research has demonstrated that in cancers like glioblastoma, a type of malignant brain tumor, cancer cells establish synapse - like connections with healthy neurons. By doing so, they tap into the neural network, stealing signal molecules that promote their growth. Breast cancer cells, too, have been found to express neural cell - surface receptors upon spreading to the brain, forming similar synaptic structures. But what sets SCLC apart is that its cancer cells can develop an independent electrical network, reducing their reliance on the surrounding neural environment, which makes the tumor even more prone to spreading.
SCLC originates from cells in the lungs with neuroendocrine functions, which are responsible for regulating lung gas exchange and blood flow. In cultured human SCLC cell lines, over 70% of the cells, known as NE cells, exhibit typical neuroendocrine characteristics. These NE cells have been identified as the primary drivers of metastasis. The current study further confirmed that NE cells not only have the genetic makeup for forming synapses but are also excitable, capable of triggering action potentials. The presence of acetylcholine receptors on both mouse and human SCLC NE cells indicates that cholinergic signals can activate their excitability.
Importantly, the electrical activity of NE cells has been linked to the high invasiveness of SCLC. In vitro experiments using tetrodotoxin (TTX), a toxin that inhibits neural electrical activity, showed that while TTX did not kill the NE cells, it significantly reduced their long - term tumor - forming ability. In in - vivo studies, suppressing the electrical activity of NE cells through chemogenetic methods effectively inhibited liver metastasis of SCLC and extended the survival of experimental animals.
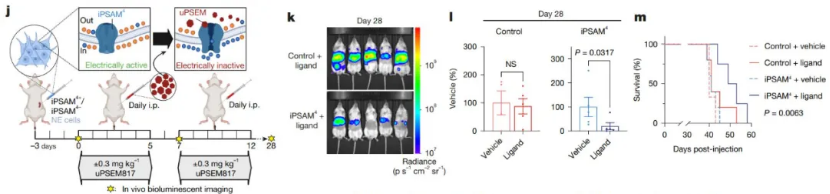
Inhibiting the electrical activity of NE cells using chemogenetic methods can suppress cancer metastasis and prolong the survival of animals
The energy - consuming nature of electrical discharges led researchers to investigate how these cancer cells meet their energy demands. They discovered that NE cells have a unique metabolic pattern. Unlike most cancer cells that rely heavily on aerobic glycolysis for energy, NE cells show an unusual dependence on oxidative phosphorylation. Additionally, non - neuroendocrine cells in the tumor secrete lactate, which sustains the high - energy requirements of NE cells, similar to the "lactate shuttle" mechanism in the brain where astrocytes support neurons.
This new understanding of SCLC's electrical and metabolic characteristics opens up new avenues for research. The researchers aim to exploit these unique features to identify vulnerabilities in the tumor, with the ultimate goal of developing novel treatment strategies. Given that SCLC serves as a model for highly metastatic cancers, insights gained from this research could potentially extend beyond SCLC, offering new hope for patients suffering from other aggressive forms of cancer.
References
[1] Paola Peinado et al., Intrinsic electrical activity drives small - cell lung cancer progression. Nature 2025, doi: https://doi.org/10.1038/s41586 - 024 - 08575 - 7
[2] Lung cancer cells can go ‘off grid’. Retrieved Feb. 13, 2025 from https://www.eurekalert.org/news - releases/1073274







Post comments