One of the major reasons why cancer is so difficult to treat is immune escape. Cancer cells will take various ways to disguise as normal cells to avoid killing by immune cells. Immunotherapy, which removes the mask of cancer cells, has rewritten the rules of treatment for many types of cancer, allowing countless patients to regain their lives. However, there are various means for cancer cells to achieve immune escape, which makes the current immunotherapy helpless in many cases.
Not long ago, by a team of researchers, published a heavy research paper in the top journal “Cell”, which opens a new way of thinking for cancer treatment.
They integrated the gene encoding porcine α1,3-galactosyltransferase (α1,3-GT) into Newcastle Disease Virus (NDV), and constructed a recombinant oncolytic virus, NDV-GT. Mechanistically, after infecting the cancer cells, NDV-GT expresses porcine α1,3-galactosyltransferase, which then synthesizes a type of glycan, αGal, that is enriched on the surface of the porcine cells, and labels the cancer cells as dissident --pig cells; naturally, hyperacute rejection ensues and the tumor is attacked by the immune system.
Of particular note, they also conducted a clinical trial on 20 patients with recurrent/refractory metastatic cancers, with eight major cancer types including common lung, breast, liver, bowel and esophageal cancers. Overall, the disease control rate reached 90%, with durable patient response and no serious adverse events.
Who would have thought that hyperacute rejection, which has caused great trouble for allogeneic organ transplants, could potentially make a big difference on the battlefield against cancer.

Screenshot of the front page of the paper
There are several reasons why the theory of hyperacute rejection should be applied to the field of antitumor therapy.
First, hyperacute rejection causes very serious damage to xenografts, activating complement-dependent cytotoxic effects (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC), resulting in graft failure; second, due to evolution, the human α1,3GT gene is inactive, but the body produces a large amount of anti-Gal antibodies, which is the reason why the body produces a hyperacute rejection response.
Before designing the recombinant lysovirus, the team first confirmed the presence of anti-Gal antibodies in both cancer patients and crab-eating monkeys. It then took the gene encoding porcine α1,3-galactosyltransferase (α1,3-GT) and integrated it into Newcastle Disease Virus (NDV) to construct the recombinant lysovirus NDV-GT.
Subsequently, they tested NDV-GT properties in a variety of cancer cell lines and normal cell lines. Initially, it was confirmed that NDV-GT could infect cancer cells and normally express α1,3-GT and synthesize αGal; whereas in healthy cells, the presence of NDV-GT and αGal was not detected. This suggests that NDV-GT specifically targets cancer cells.
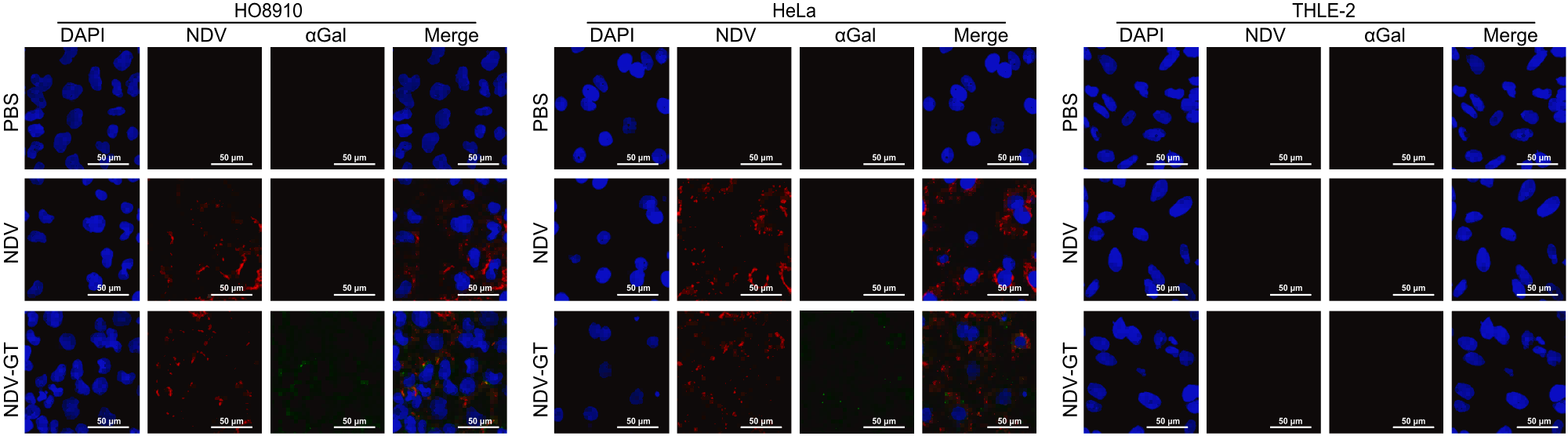
NDV-GT (red) will specifically target cancer cells (HO8910 and Hela) without infecting healthy cells (THLE-2)
In terms of cancer inhibitory activity, NDV-GT had a stronger inhibitory effect on hepatocellular carcinoma cell line (HepG2) compared to NDV. The inhibition rate was about 90% after 72 hours of culture.
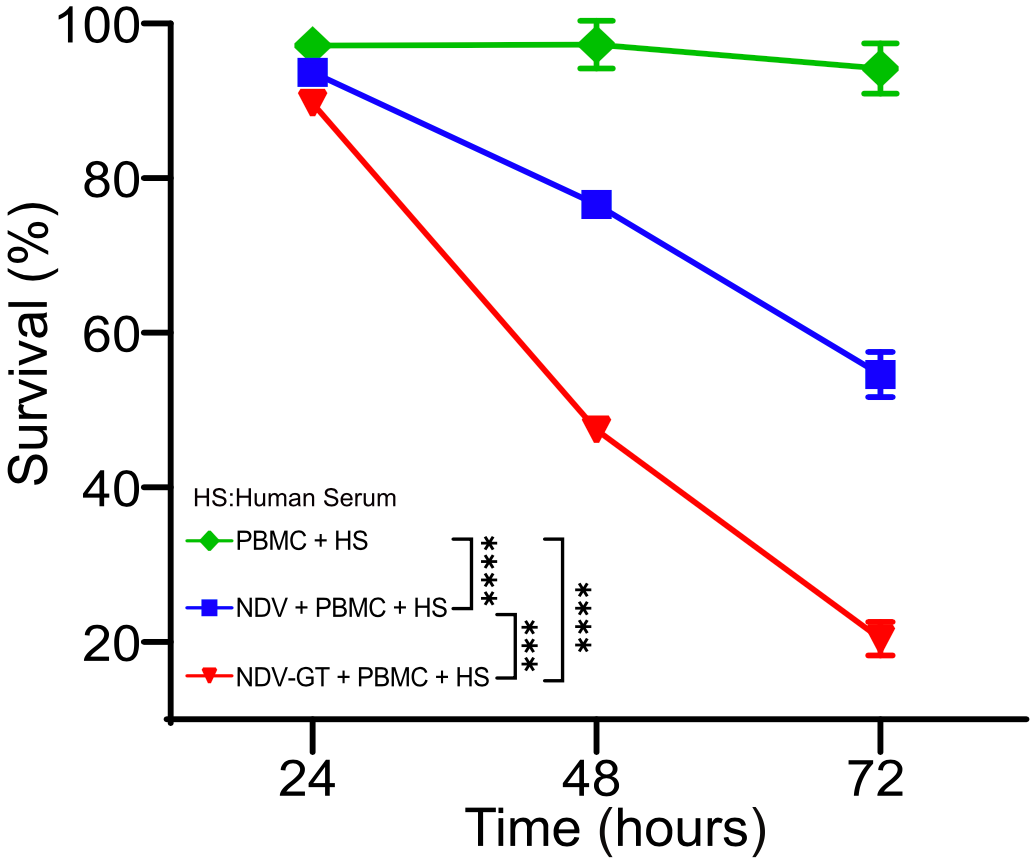
Strong cancer inhibitory activity of NDV-GT
On the basis of the cellular studies described above, the team began to test the effects of NDV-GT in a crab-eating monkey model of hepatocellular carcinoma.
After intravenous injection of NDV-GT, tumor tissue was biopsied, stained and analyzed by immunofluorescence at 0, 24, 48, 72 and 96 hours.
Overall, after receiving three months of treatment, the tumors in the NDV-GT group of crab-eating monkeys were significantly smaller than those in the NDV and PBS groups. Notably, the tumors of the swollen crab monkeys in the NDV-GT group completely disappeared three months after cessation of treatment, and all monkeys survived for more than 6 months. two monkeys in the NDV group died due to tumor recurrence after cessation of treatment, whereas the monkeys in the PBS group survived for as short as 3 months and as long as more than 5 months.
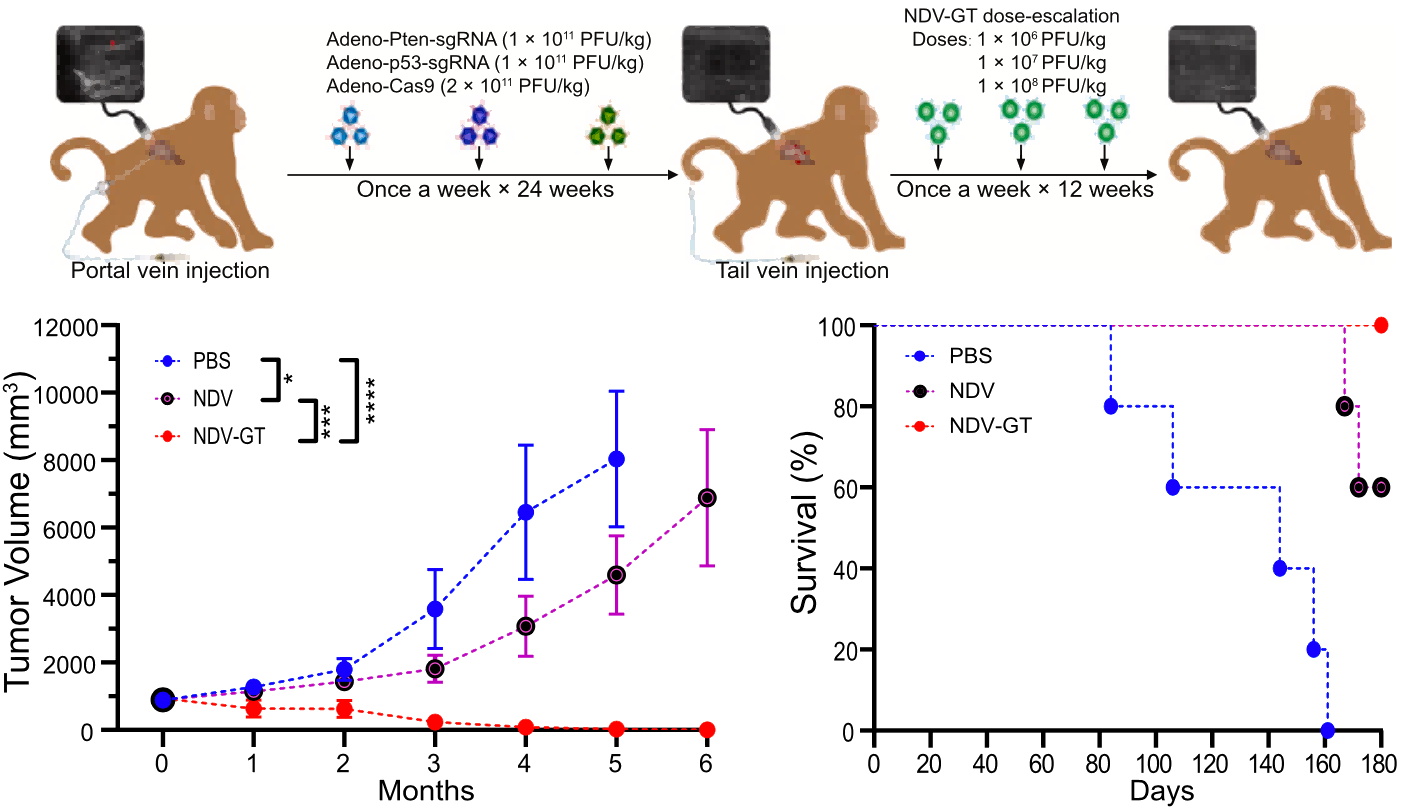
NDV-GT has some anti-tumor effects
From the anatomical findings, there were signs of thrombosis within the tumor due to vascular atrophy and occlusion following NDV-GT treatment.
The researchers concluded that NDV-GT treatment allowed the cancer cells to form αGal, which binds to the natural anti-Gal antibodies within the tumor vasculature, which in turn triggers a hyper-acute rejection reaction that leads to platelet activation and aggregation. Together, these processes promote thrombosis and vascular occlusion, ultimately leading to tumor shrinkage and necrosis.
Based on immunofluorescence staining of complement, Yongxiang Zhao's team also found that complement activation was indeed involved in the hyperacute rejection reaction. In addition, they noted that the expression of granzyme B and perforin was significantly elevated in NDV-GT-infected tumor tissues, suggesting that antigen-triggered anti-tumor immune responses recruited and promoted T-cell infiltration.
Based on these findings, the team concluded that recombinant tumor lysing virus NDV-GT can directly lyse tumor cells, destroy intratumor blood vessels, and promote αGal synthesis, leading to hyperacute rejection and thrombosis. The above changes will improve the immunosuppressive microenvironment of the tumor, activate humoral and cellular immunity, promote T-cell infiltration, and expand the anti-tumor cascade immune response.
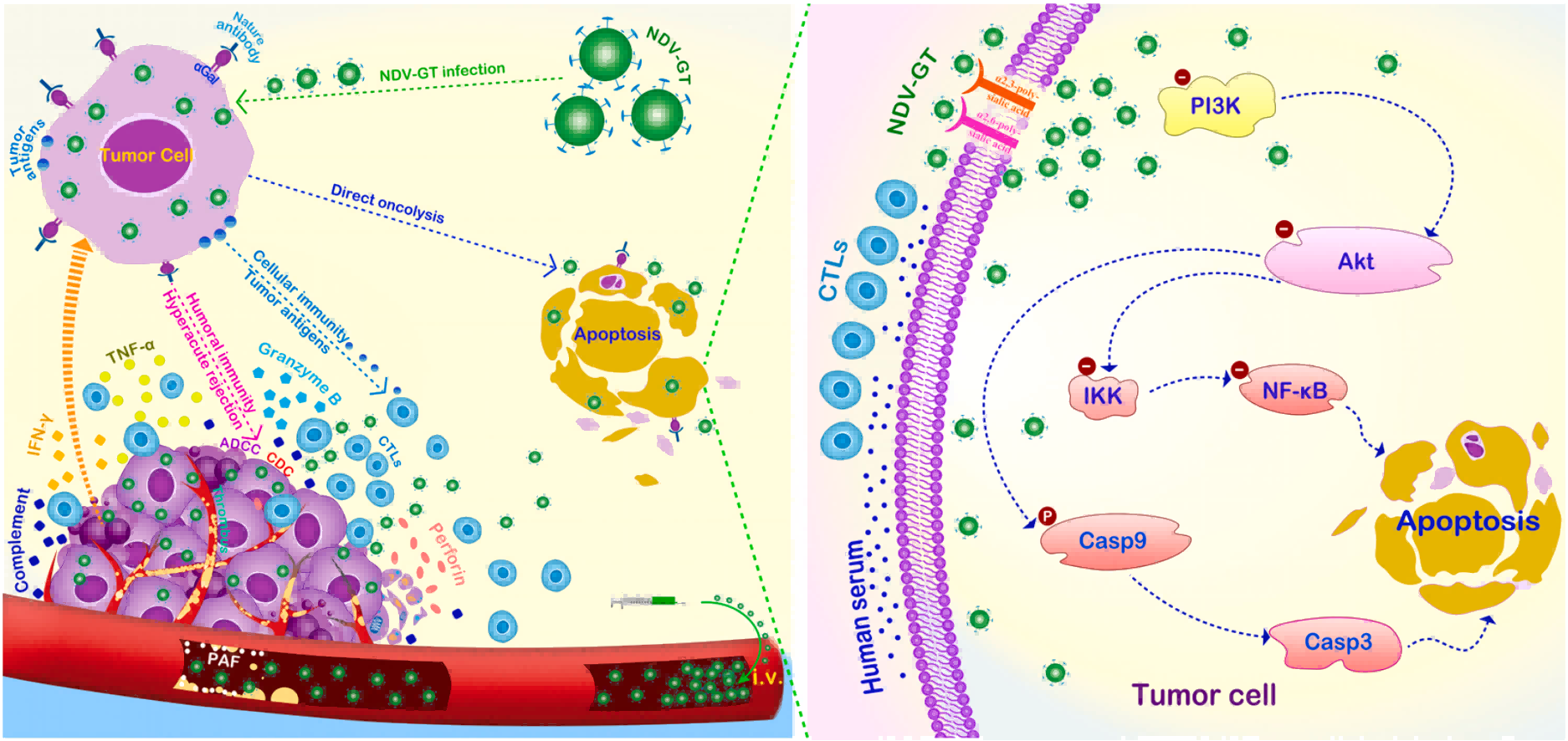
Schematic diagram of the anti-cancer mechanism of NDV-GT
As for safety, after analyzing various tissues of the crab-eating monkeys treated with NDV-GT, the team confirmed that the NDV-GT treatment did not affect the body weight, body temperature and heart rate of the crab-eating monkeys, and that the effects on their physiological indexes, coagulation factors, liver and kidney functions, and blood glucose levels were minimal. In addition, there were no changes in urinary proteins, leukocytes and fecal occult blood, and there was no significant damage to the healthy organs of the monkeys.
On the basis of the above study, the team initiated a clinical study (ChiCTR2000031980) that recruited a total of 23 patients with refractory cancers, including 3 patients with liver cancer, 6 patients with ovarian cancer, 3 patients with bowel cancer (2 withdrew from the study due to the New Crown Pandemic), 3 patients with lung cancer (1 withdrew from the study due to the New Crown Pandemic), 2 patients with breast cancer, 1 esophageal cancer patient, 1 melanoma patient, and 4 cervical cancer patients.
Twenty patients eventually completed the clinical trial, with 1 patient achieving complete remission (CR), 6 patients partial remission (PR), and 11 patients stable disease (SD), for an overall disease control rate of 90% (18/20). There were no significant toxic reactions during the treatment period. This result tentatively confirms that NDV-GT treatment may be safe and effective for cancer patients.
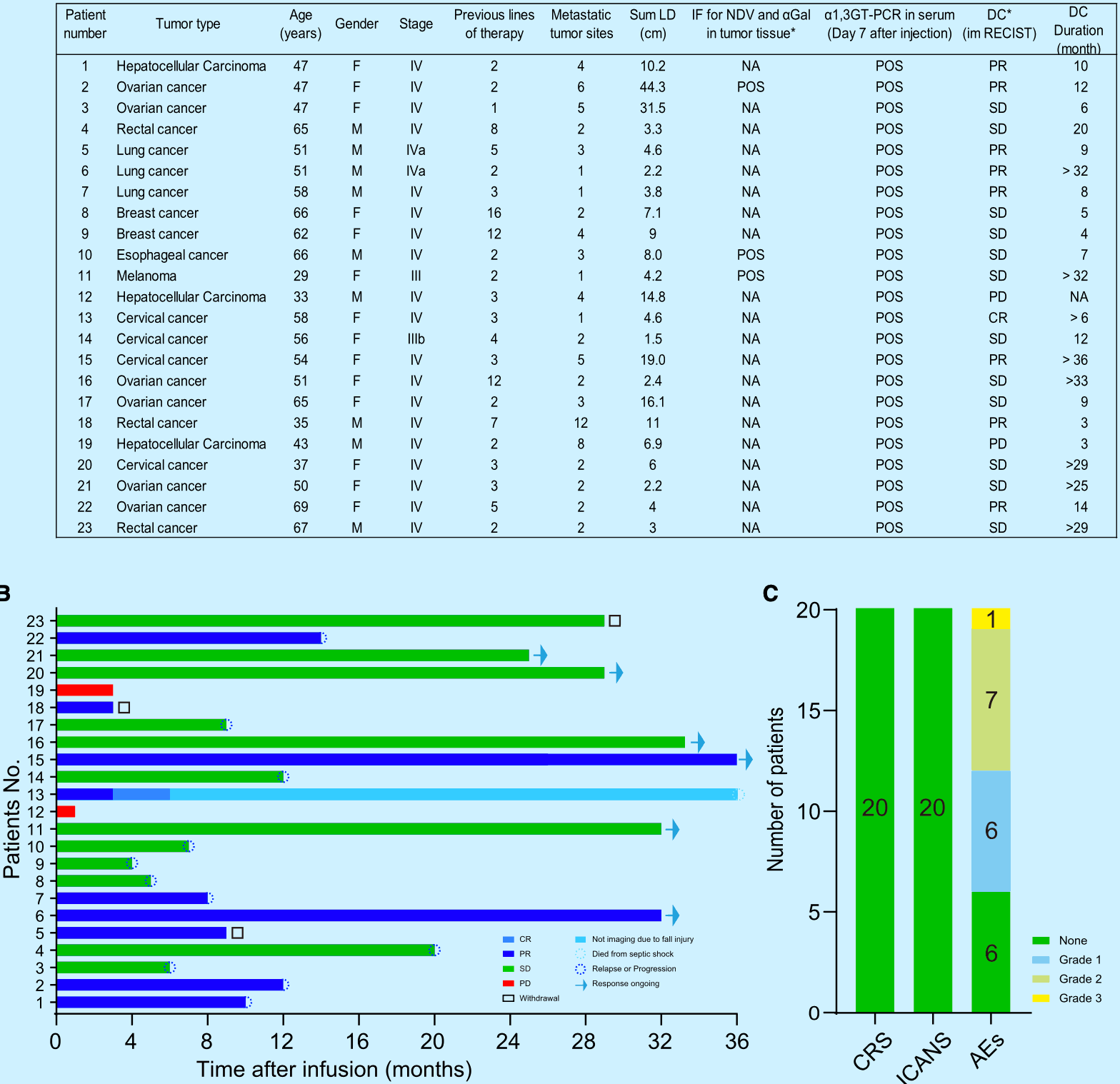
Basic profile of the 23 patients
Overall, the team's research results innovatively apply the theory of hyperacute rejection in reverse to the field of antitumor, develop a novel broad-spectrum oncolytic virus therapy, and preliminarily demonstrate the efficacy and safety of NDV-GT in early clinical studies.
Of course, the efficacy and safety of NDV-GT have yet to be confirmed in larger clinical studies. In any case, we look forward to seeing more cancer patients benefit from this innovative anti-cancer therapy that turns tumors into “pork”.








Post comments