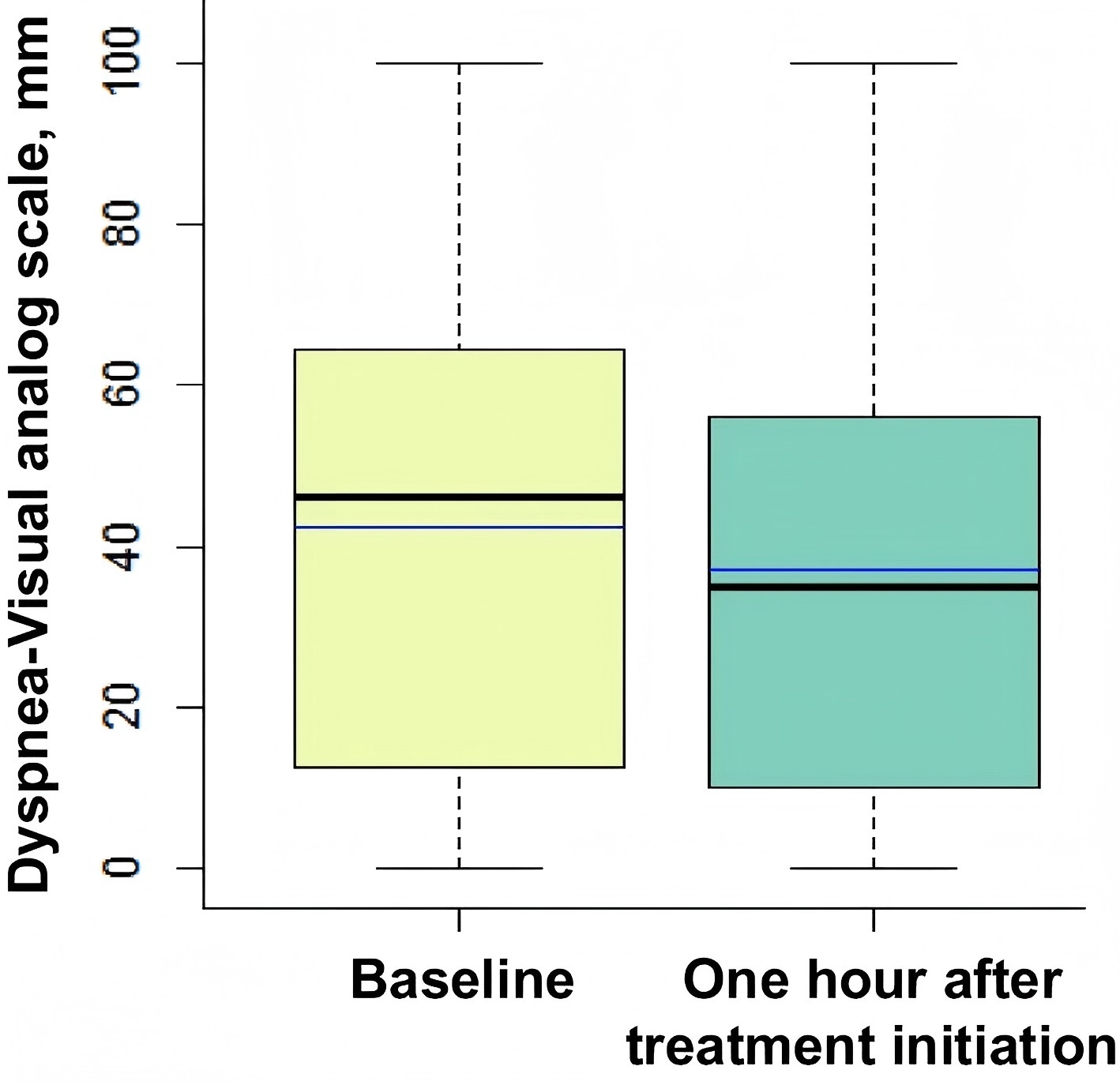
Box plots showing dyspnea-visual analog scale from zero (no respiratory discomfort) to 100 mm (worst imaginable respiratory discomfort) at baseline and 1 h after treatment initiation. The black center line denotes the median value (50th percentile), while the box contains the 25th to 75th percentiles of dataset. The blue line denotes the mean value. The black whiskers mark the maximal and minimal values
Acute hypoxemic respiratory failure is a common and severe pathological condition, typically characterized by dyspnea and hypoxemia, with many patients requiring intubation and mechanical ventilation for support. Although dyspnea is widely recognized as a clinical symptom, its quantitative assessment and predictive role in clinical outcomes remain inadequately validated. To address this, Demoule et al. conducted a secondary analysis of a multicenter, randomized controlled trial involving 259 patients with acute hypoxemic respiratory failure. At enrollment, patients' dyspnea was quantified using the visual analog scale (dyspnea-VAS), with scores ranging from 0 (no dyspnea) to 100 (extreme dyspnea). The study analyzed factors associated with intubation using a competing risks model and evaluated the association with 90-day mortality using a Cox regression model, aiming to determine whether the quantitative assessment of dyspnea could serve as a significant predictor of intubation requirements and mortality.
At baseline, the median dyspnea score was 46 (interquartile range: 16–65), and 45% of patients required intubation. The results showed that patients with baseline dyspnea scores of 40–64 mm (moderate dyspnea) and ≥65 mm (severe dyspnea) had relative risks of 1.96 and 2.61 for requiring intubation, respectively, with both results being statistically significant (p = 0.023). These findings suggest that the severity of dyspnea is an important predictor of intubation requirements in patients with acute hypoxemic respiratory failure.
Additionally, the study found that during the 90-day follow-up, patients with a dyspnea score ≥40 mm had a significantly lower cumulative survival probability compared to those with a score <40 mm (Log-rank test, p = 0.049). This suggests that a higher dyspnea score is significantly associated with poorer survival outcomes. Further Cox regression analysis identified independent predictors of mortality, including the SAPS II score (p < 0.001), baseline moderate-to-severe dyspnea (dyspnea-VAS ≥40 mm, p = 0.073), and changes in the oxygenation index (PaO₂/FiO₂). These findings indicate that the quantitative assessment of dyspnea is not only closely related to intubation requirements but also significantly associated with short-term mortality, suggesting its potential as a valuable prognostic marker.
This study is the first to systematically combine the quantitative dyspnea score with intubation requirements and mortality, underscoring the potential of dyspnea as a clinical prognostic tool. Unlike previous studies, which primarily focused on oxygenation status (e.g., PaO₂/FiO₂ ratio) and clinical scoring systems (such as APACHE or SAPS scores) for predicting mortality, this study introduces a new, more personalized clinical decision-support tool through the quantitative dyspnea score. This innovation is significant not only for the early prediction of intubation needs and mortality risk but also for providing new evidence for the development of personalized treatment strategies.
However, despite using the VAS, a standardized tool, the assessment of dyspnea remains inherently subjective, as it relies on self-reported evaluations. This may be influenced by factors such as individual emotions, psychological state, understanding of the illness, and even environmental conditions. Furthermore, in clinical practice, the relationship between intubation and mortality is complex, and patients' conditions may change depending on the treatment strategy employed. Therefore, it is important to critically assess whether all assumptions regarding competing risks have been fully validated within the model.
It is also worth noting that while this study used dyspnea scores to predict intubation rates and mortality, it did not explore the specific effects of different treatment strategies on these outcomes. Future research could investigate combining other objective physiological data (such as chest imaging, blood gas analysis, and pulmonary ventilation function) with dyspnea scores to develop more comprehensive and accurate prognostic tools. Additionally, incorporating treatment strategies as a variable in the analysis could provide valuable insights into how different treatments impact outcomes at varying levels of dyspnea severity. Specifically, examining whether optimizing treatment approaches according to dyspnea severity can improve clinical outcomes would offer more personalized and precise guidance for clinical treatment.
REFERENCES:Demoule A, Baptiste A, Thille A W, et al. Dyspnea is severe and associated with a higher intubation rate in de novo acute hypoxemic respiratory failure[J]. Critical Care, 2024, 28(1): 174.







Post comments