by TranSpread
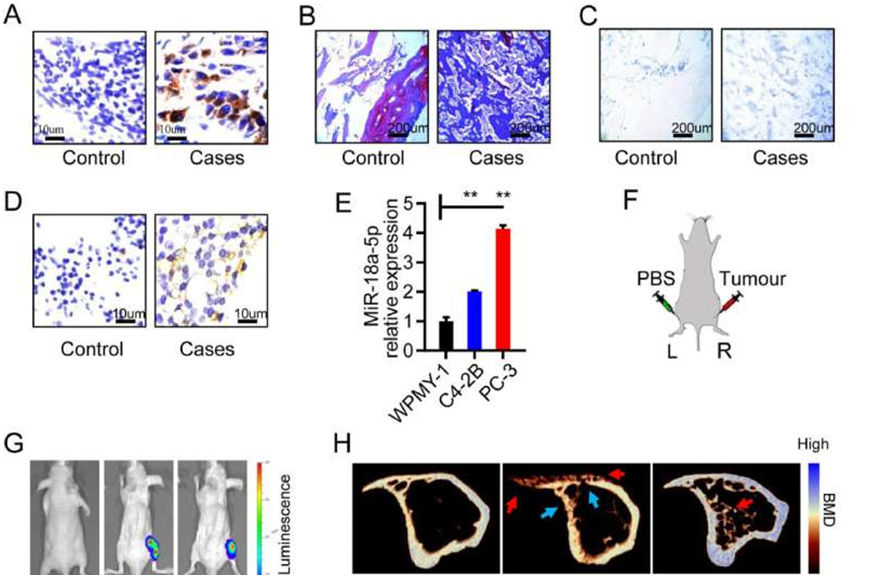 MiR-18a-5p was overexpressed in osteoblastic lesions of PCa bone metastasis. (A–D) Bone specimens of PCa patients with bone metastasis. (A) Immunohistochemical labeling of PSA (brown represents positive). (B) Masson staining (blue represents young bone, red represents old bone). (C) TRAP staining (purple represents positive). (D) In situ hybridization of miR-18a-5p (brown represents positive). (E) The miR-18a-5p expression in prostate cells and PCa bone metastasis cells. (F) Schematic diagram of the PCa bone metastasis model in nude mice (L represents left tibia; R represents right tibia). (G) In vivo imaging of nude mice implanted with PBS or PCa cells for 4 weeks. (H–K) Tibia specimens of PCa bone metastasis in nude mice after PCa cells implantation for 4 weeks. (H) Representative micro-CT 3D-reconstruction models illustrating in tibia (red arrows indicates an osteoblastic lesion; blue arrows indicate an osteolytic lesion). (I) The ultimate load of tibia implanted with C4-2B cells. (J) The Young's modules of tibia implanted with C4-2B cells. (K) In situ hybridization of miR-18a-5p (brown represents positive). The data are represented as mean ± SEM or SD. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ns P > 0.05. Credit: Genes & Diseases (2022). DOI: 10.1016/j.gendis.2022.06.007
MiR-18a-5p was overexpressed in osteoblastic lesions of PCa bone metastasis. (A–D) Bone specimens of PCa patients with bone metastasis. (A) Immunohistochemical labeling of PSA (brown represents positive). (B) Masson staining (blue represents young bone, red represents old bone). (C) TRAP staining (purple represents positive). (D) In situ hybridization of miR-18a-5p (brown represents positive). (E) The miR-18a-5p expression in prostate cells and PCa bone metastasis cells. (F) Schematic diagram of the PCa bone metastasis model in nude mice (L represents left tibia; R represents right tibia). (G) In vivo imaging of nude mice implanted with PBS or PCa cells for 4 weeks. (H–K) Tibia specimens of PCa bone metastasis in nude mice after PCa cells implantation for 4 weeks. (H) Representative micro-CT 3D-reconstruction models illustrating in tibia (red arrows indicates an osteoblastic lesion; blue arrows indicate an osteolytic lesion). (I) The ultimate load of tibia implanted with C4-2B cells. (J) The Young's modules of tibia implanted with C4-2B cells. (K) In situ hybridization of miR-18a-5p (brown represents positive). The data are represented as mean ± SEM or SD. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ns P > 0.05. Credit: Genes & Diseases (2022). DOI: 10.1016/j.gendis.2022.06.007
In a study published in the journal Genes & Diseases, researchers from Army Medical University and Shenzhen University investigated the pivotal role of miR-18a-5p, a microRNA, in the development and progression of osteoblastic lesions resulting from prostate cancer (PCa) bone metastasis.
They made a striking observation of significantly elevated miR-18a-5p expression in the bone microenvironment of PCa patients with bone metastases, indicating its potential involvement in the pathogenesis of the disease. To gain deeper insights into the impact of miR-18a-5p on osteoblastic lesions, the researchers conducted a series of comprehensive laboratory experiments.
By inhibiting miR-18a-5p in both PCa cells and pre-osteoblasts, they successfully demonstrated a substantial reduction in osteoblast differentiation and activity. Particularly noteworthy was the administration of PCa cells with suppressed miR-18a-5p into a mouse model, which resulted in remarkable improvements in bone biomechanical properties and bone mineral mass, effectively highlighting the therapeutic potential of targeting this specific microRNA.
Subsequent investigations unraveled the intricate molecular mechanism underlying the osteoblastic lesions induced by miR-18a-5p. The researchers discovered that this microRNA was transferred to osteoblasts via exosomes secreted by PCa cells. Within the osteoblasts, miR-18a-5p skillfully targeted the Hist1h2bc gene, leading to the up-regulation of Ctnnb1 in the Wnt/β-catenin signaling pathway, ultimately driving osteoblast differentiation and fostering the formation of osteoblastic lesions.
The findings of this study hold promise for the development of novel and targeted therapeutic strategies for managing PCa bone metastasis and its associated osteoblastic complications. The researchers effectively employed antagomir-18a-5p, an inhibitor of miR-18a-5p, to ameliorate osteoblastic lesions in the mouse model, without adversely affecting osteoclast activity.
Remarkably, antagomir-18a-5p treatment significantly improved bone biomechanical properties, bone mineral density, and alleviated sclerotic lesions, underscoring its potential efficacy as a promising treatment option for PCa-induced osteoblastic lesions in clinical settings.
Given that prostate cancer bone metastasis represents a significant unmet medical need, especially concerning osteoblastic lesions, current treatments predominantly address osteolytic complications, leaving limited therapeutic options for osteoblastic manifestations. This research opens up new and exciting possibilities for targeted therapies that have the potential to significantly enhance the quality of life for PCa patients grappling with osteoblastic lesions.
The study's findings offer exciting prospects, but further research is needed to validate the safety and efficacy of this approach in humans. Nevertheless, it marks a significant step forward in combating the devastating impact of PCa bone metastasis and its osteoblastic lesions. With advancements in targeted therapies and drug delivery methods, this research could lead to improved treatments for PCa bone metastasis, bringing hope to patients, families, and health care professionals in the fight against prostate cancer and its complications.







Post comments