In the dynamic field of cancer research, patient-derived organoids (PDOs) have emerged as a groundbreaking approach, promising to transform our understanding and treatment of this complex disease. These miniature three-dimensional tissue cultures, grown from a patient’s own cells, represent a paradigm shift in cancer study, diagnosis, and treatment. PDOs offer an unprecedented level of personalization in cancer research, capturing the unique genetic and cellular characteristics of each patient’s tumor. This specificity allows researchers and clinicians to model tumor environments with exceptional accuracy, enhance drug testing protocols, and develop truly personalized treatment strategies.
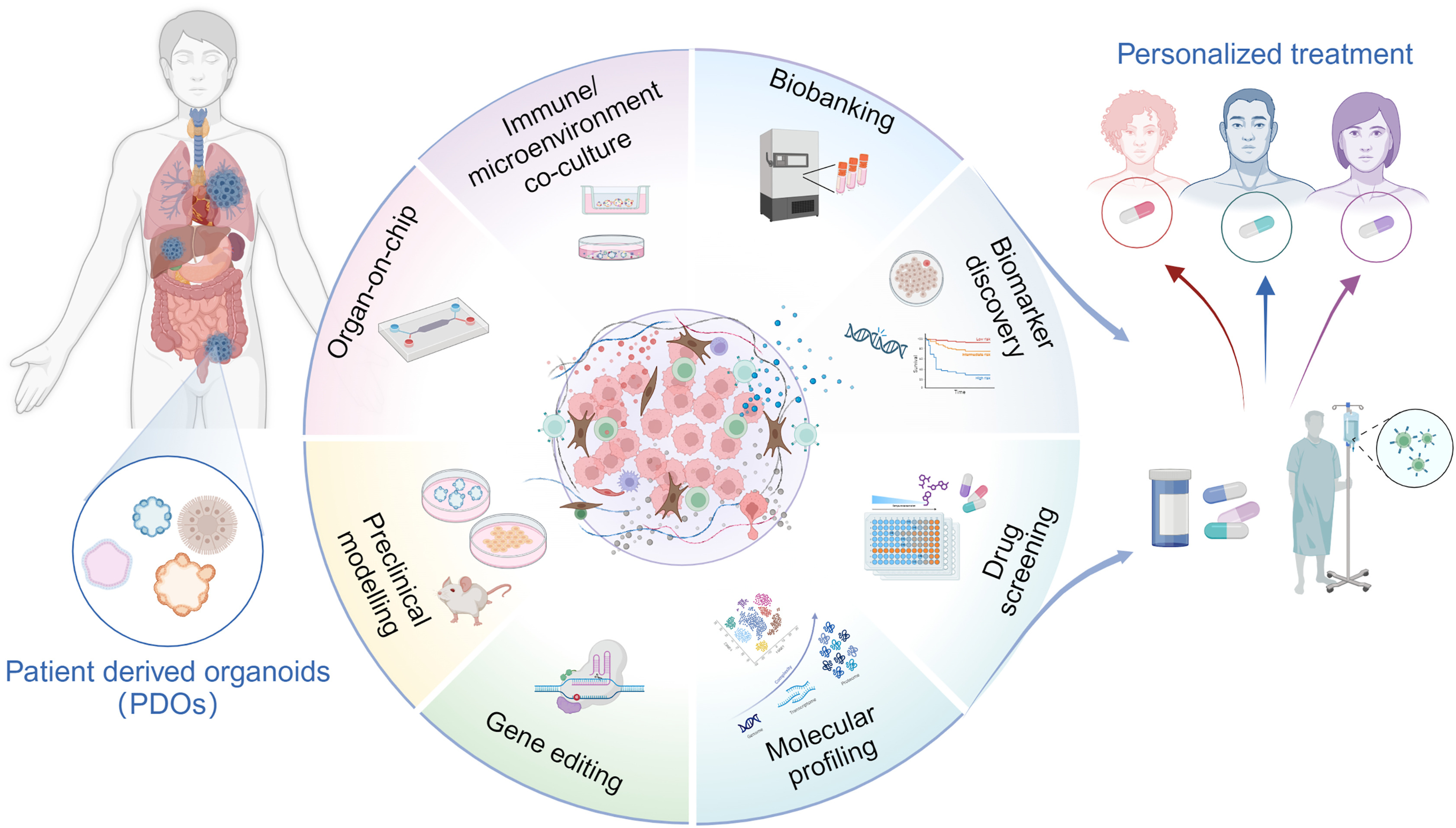
The power of PDOs lies in their ability to replicate the intricate complexity of human tumors, including the tumor microenvironment (TME), cellular heterogeneity, and interactions between cancer cells and surrounding tissues. By faithfully reproducing these aspects, PDOs serve as invaluable preclinical models for gene editing experiments, molecular profiling, drug testing, and biomarker discovery – crucial steps towards realizing the promise of personalized cancer treatment. PDOs excel in simulating the intricate interactions between cancer cells and their environment. Researchers have developed sophisticated co-culture models that allow for the study of cancer and immune cell interactions, providing insights into mechanisms of drug resistance and the efficacy of immunotherapy approaches. Co-cultures of cancer-associated fibroblasts (CAFs) and PDOs reveal how CAFs contribute to tumor progression and drug resistance, while endothelial cell (EC)-PDO co-cultures illuminate processes of angiogenesis and inflammatory responses in the tumor microenvironment.
The field of PDO research is rapidly advancing, with new techniques emerging to enhance their utility. Air-liquid interface (ALI) cultures support 3D tumor growth, maintain functional immune cells, and allow assessment of immune checkpoint blockade therapies. Microfluidic cultures enable the sustenance of both tumor and immune cells in dynamic environments, facilitating high-throughput drug screening and personalized immunotherapy development. Organ-on-Chip (OoC) technology replicates tumor physiology using microfluidics, enabling the study of resistance mechanisms and drug response analysis.
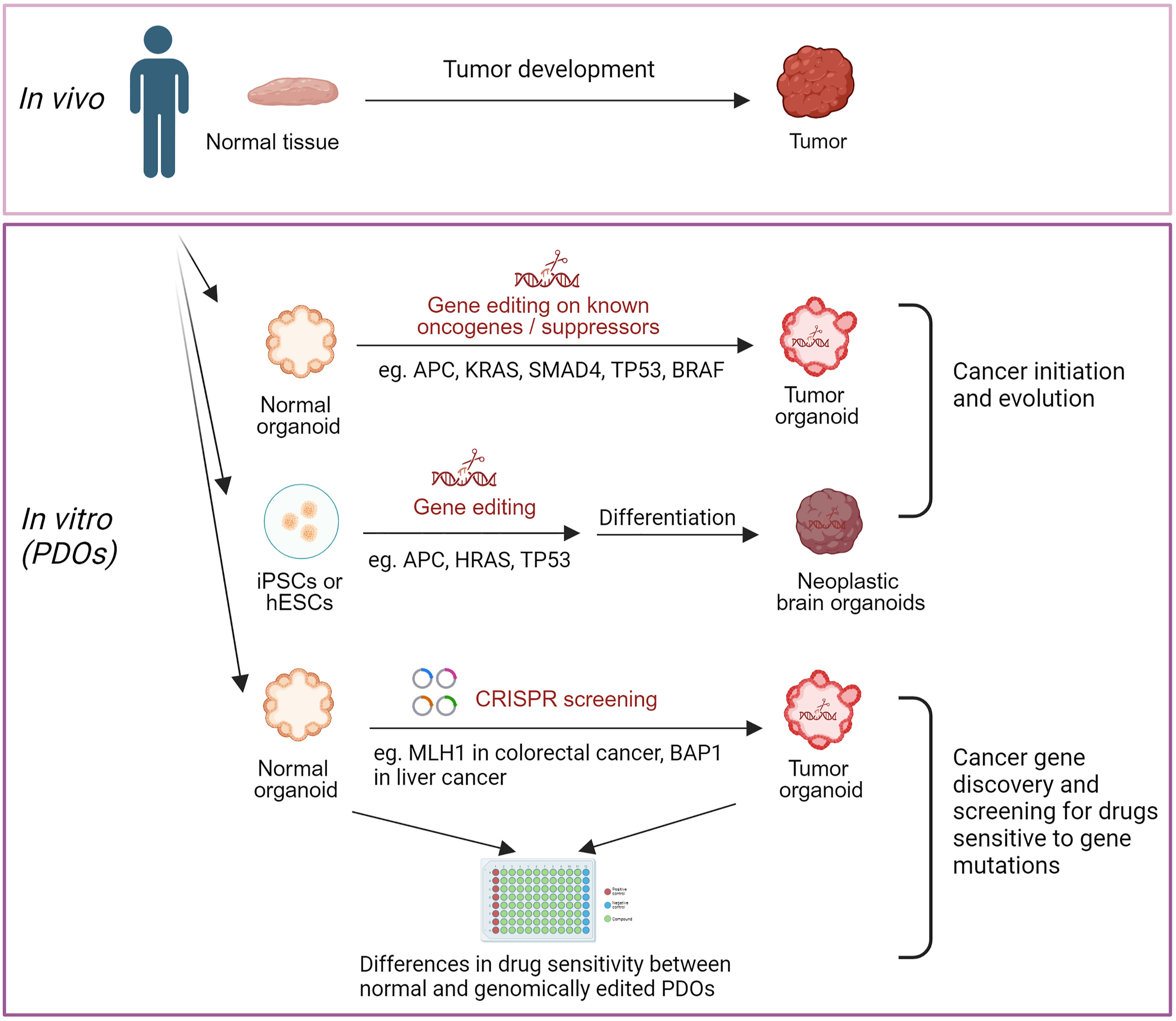
PDOs derived from stem cells are proving invaluable in studying cancer heterogeneity. Advanced gene editing tools allow researchers to target specific oncogenes, investigate tumor progression markers, and identify and study tumor-specific mutations. Molecular profiling of PDOs offers insights that go beyond the limitations of traditional biopsies, providing a more comprehensive view of the tumor’s genetic landscape. One of the most promising applications of PDOs is in drug screening and biomarker discovery. PDOs can mimic patient-specific genetic diversity, assist in predicting drug responses, and guide treatment decisions for resistant cancers. Advances in spatial transcriptomics are enhancing the capability of PDOs in high-throughput drug screening, allowing for more efficient and accurate testing of potential therapies. PDOs also aid in discovering new biomarkers, revealing mechanisms of treatment efficacy, and understanding drug resistance pathways.
The establishment of PDO biobanks for various cancer types represents a significant step forward. These biobanks support personalized therapy development, facilitate drug research on a broader scale, and hold transformative potential for regenerative medicine. While challenges remain, such as optimizing maturation processes and providing adequate structural support, the potential of these biobanks in advancing cancer treatment is immense.
Patient-derived organoids stand at the intersection of cutting-edge biology and personalized medicine. As physiologically relevant, high-throughput models, they offer unparalleled opportunities for biomarker discovery, drug development, and tailoring treatments to individual patients. With ongoing advancements in 3D culturing techniques, gene editing technologies, and computational tools, PDOs are poised to become an essential and cost-effective resource in cancer care. They pave the way for innovative, patient-specific therapies that could revolutionize our approach to treating this complex and devastating disease. As we look to the future, the integration of PDOs into clinical practice promises to bridge the gap between laboratory research and bedside care, offering hope for more effective, personalized cancer treatments. The era of truly precision oncology, powered by these miniature marvels, is on the horizon.
Source: https://www.cell.com/med/fulltext/S2666-6340(24)00343-X






Post comments